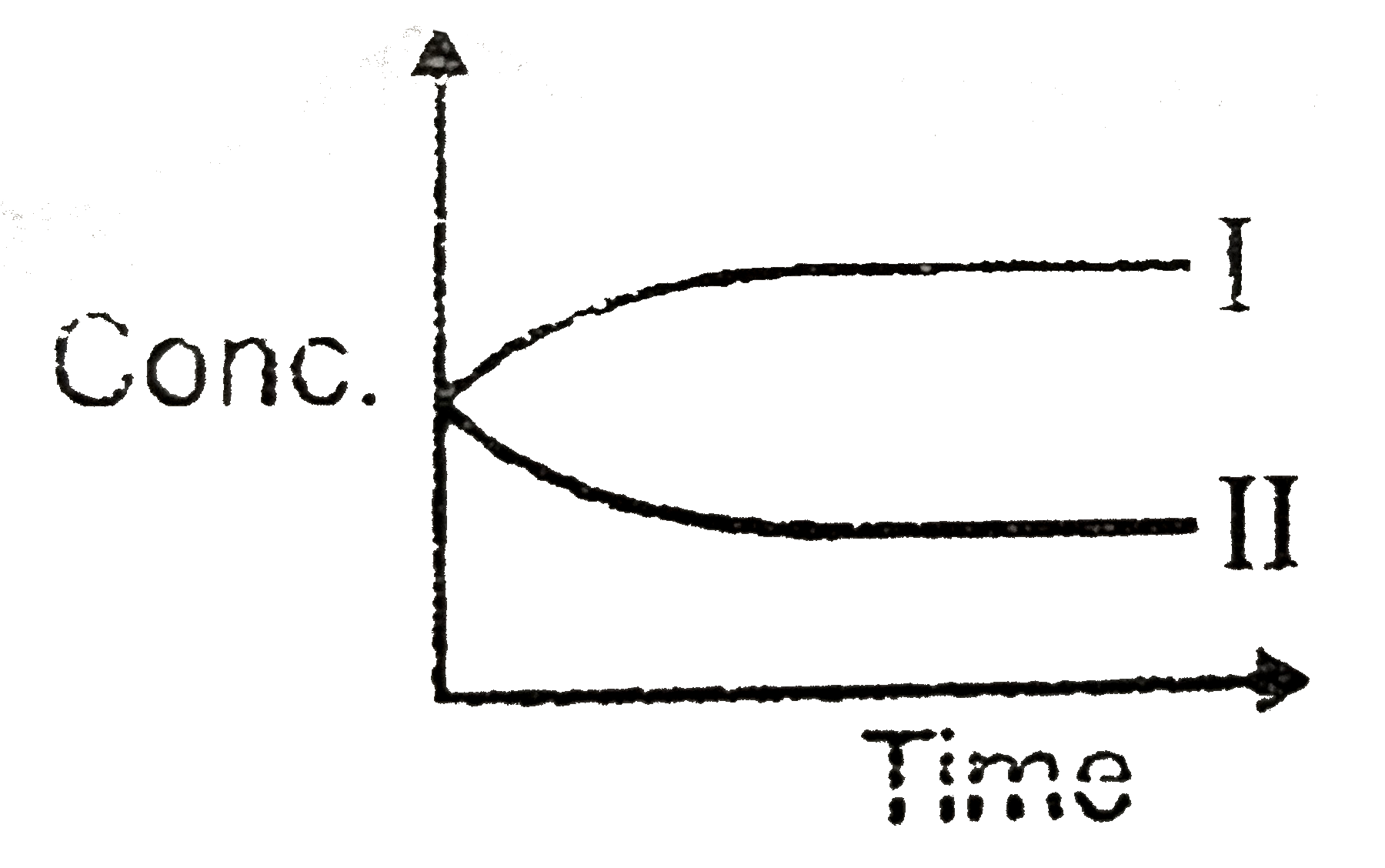
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL EQUILIBRIUM
RESONANCE ENGLISH|Exercise Exercise-2 (Part-4)|6 VideosCHEMICAL EQUILIBRIUM
RESONANCE ENGLISH|Exercise Exercise-3 (Part-1)|6 VideosCHEMICAL EQUILIBRIUM
RESONANCE ENGLISH|Exercise Exercise-2 (Part-2)|22 VideosCHEMICAL BONDING
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Fundamental Concept )|6 VideosD & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS
RESONANCE ENGLISH|Exercise Match the column|1 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-CHEMICAL EQUILIBRIUM-Exercise-2 (Part-3)
- At 300 K, the reactions satisfying the following graph is:
Text Solution
|
- N(2)O(4)(g)hArr2NO(2)(g),K(C)=4. This reversible reaction is studied g...
Text Solution
|
- A 2 lit vessel is filled by 1 mole of each gas A "&" B. If K(C) for re...
Text Solution
|
- The equilibrium constant for some reactions are given below against ea...
Text Solution
|
- For the reaction, SnO(2)(s)+2H(2)(g) hArr Sn(l)+2H(2)O(g) the equilibr...
Text Solution
|
- The equation alpha=(D-d)/((n-1)d) is correctly matched for: (alpha is ...
Text Solution
|
- If reaction A+BhArrC+D, taken place in 5 liter close vessel, the rate ...
Text Solution
|
- Consider equilibrium H(2)O(l)hArrH(2)O(g). Choose the correct directio...
Text Solution
|
- Consider the following equilibrium 2AB(g)hArrA(2)(g)+B(2)(g) The v...
Text Solution
|
- Vapour density of equilibrium PCI(5)(g)hArrPCI(3)(g)+CI(2)(g) is decre...
Text Solution
|
- CuSO(4),5H(2)O(s)hArrCuSO(4)(s)+5H(2)O(g)K(P)=10^(-10) "moles of" CuSO...
Text Solution
|
- CuSO(4).5H(2)O(s)hArrCuSO(4).3H(2)O(s)+2H(2)O(s) K(P)=0.4xx10^(-3) atm...
Text Solution
|
- Each question contains STATEMENT-1 (Assertion) and STATEMENT-2( Reason...
Text Solution
|
- 1 mole each of H(2)(g) "and" I(2)(g) are introduced in a 1L evacuated ...
Text Solution
|
- For the reaction PCl(5(g))hArrPCl(3(g))+Cl(2(g)), the forward reactio...
Text Solution
|
- Which of the following reaction will shift in forward direction. When ...
Text Solution
|
- Which of the following will not affect the value of equilibrium consta...
Text Solution
|
- If the volume of the racion flask is reduced to half of its initial va...
Text Solution
|
- 2CaSO4 (s) hArr 2CaO(s)+2SO(2)(g)+O2(g), DeltaHgt0 Above equilibrium...
Text Solution
|
- An industrial fuel, 'water gas', which consists of a mixture of H(2) "...
Text Solution
|