A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
AROMATIC COMPOUNDS
RESONANCE ENGLISH|Exercise PART-II: NATIONAL STANDARD EXAMINATION IN CHEMISTRY (NSEC) STAGE-I|31 VideosAROMATIC COMPOUNDS
RESONANCE ENGLISH|Exercise PART-III: PRACTICE TEST-2(IIT-JEE(ADVANCED pattern))|7 VideosAROMATIC COMPOUNDS
RESONANCE ENGLISH|Exercise JEE(MAIN) ONLINE PROBLEM|16 VideosATOMIC STRUCTURE
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Fundamental Concept )|16 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-AROMATIC COMPOUNDS -PART-I PRACTICE TEST-1(IIT-JEE MAIN PATTERN)
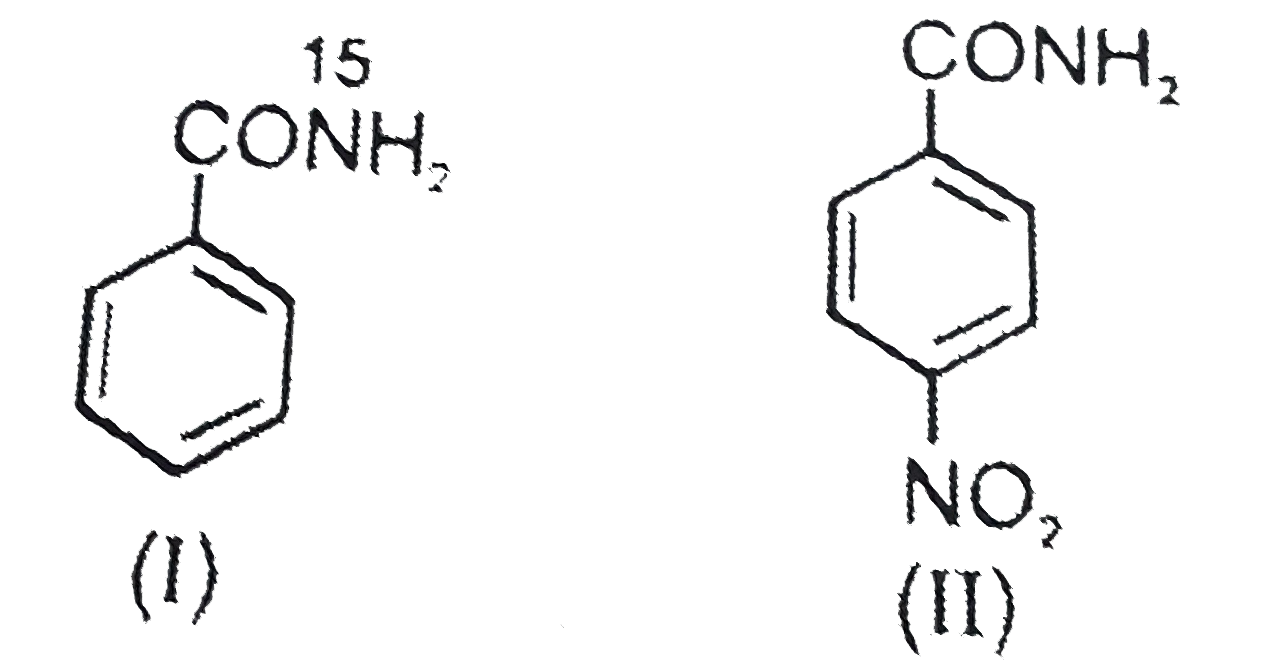
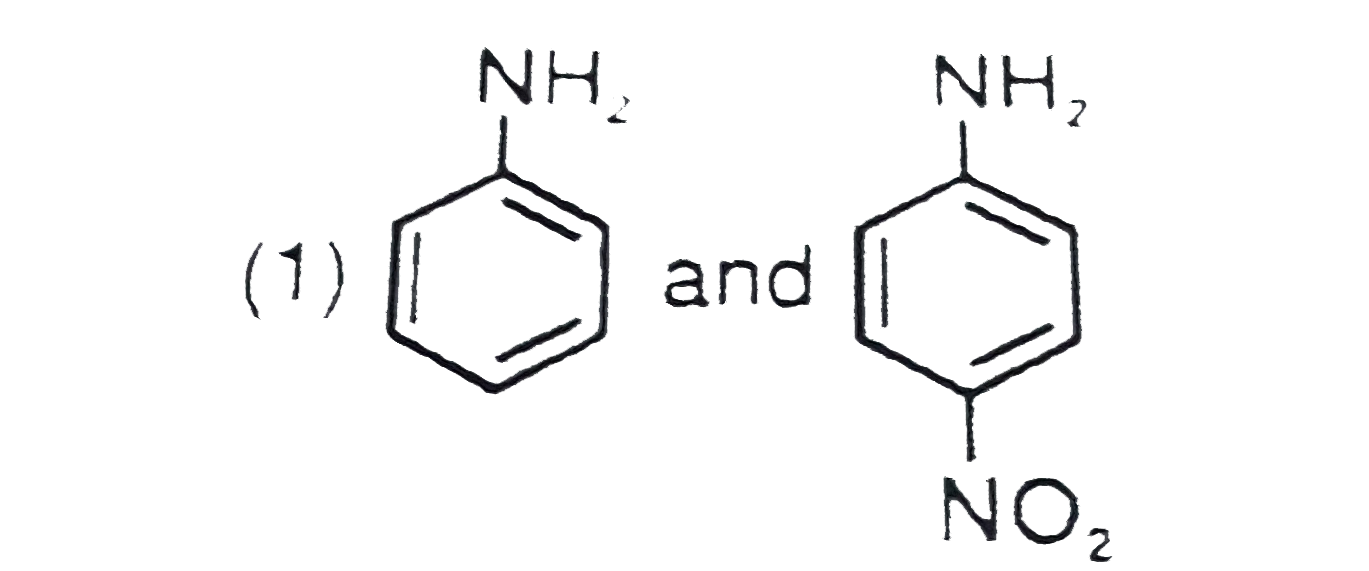
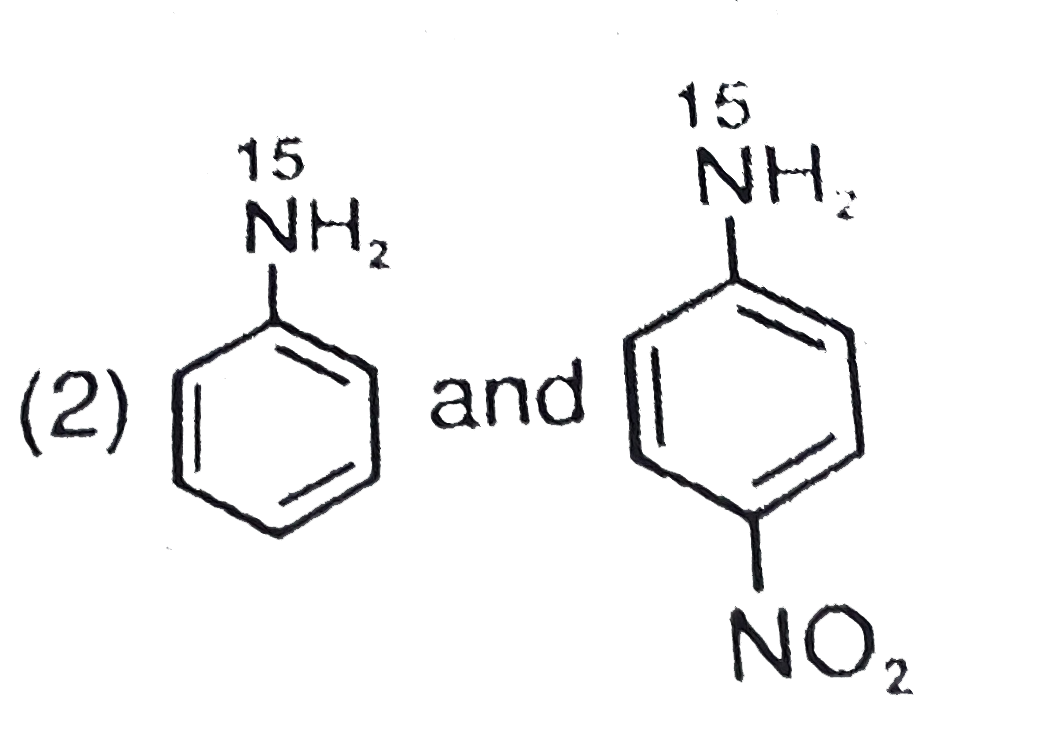
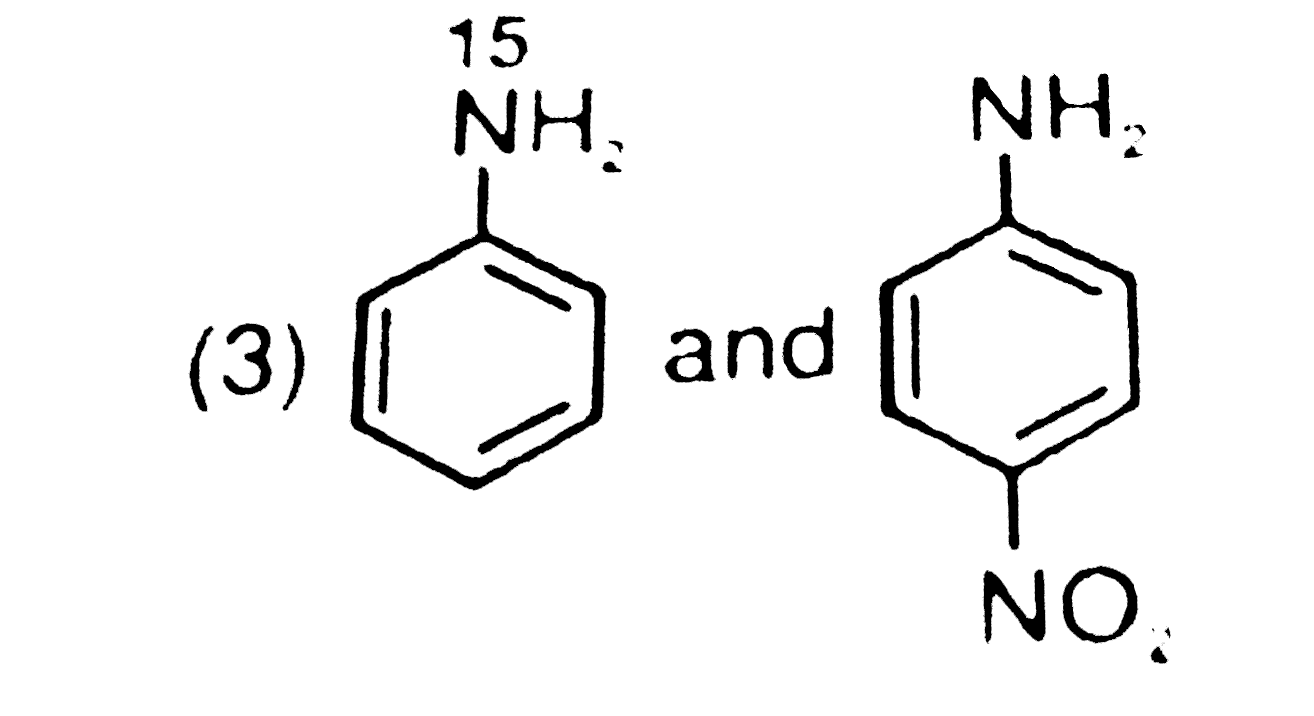
- The products formed when a mixture of the following two amides( I and ...
Text Solution
|
- Which of the following does not give effervescence with NaHCO(3) ?
Text Solution
|
- The compound which does not give foul smell when heated with CHCl(3) a...
Text Solution
|
- The final product C, obtained in this reaction would be
Text Solution
|
- The action of bromine water (excess) on salicylic acid results in the ...
Text Solution
|
- 'S' is:
Text Solution
|
- Electrolytic reduction of nitrobenzene in weakly acidic medium gives :...
Text Solution
|
- In the following reaction , B is Aoverset("Bromination")toBoverset(...
Text Solution
|
- Predict the product,
Text Solution
|
- Complete the following reaction
Text Solution
|
- Reaction by which benzaldehyde cannot be prepared?
Text Solution
|
Text Solution
|
- The reaction of chloroform with alcoholic KOH and p-toluidine form-
Text Solution
|
- An organic compound A on reduction gives compound B which on reaction ...
Text Solution
|
- Primary amine reacts with carbon disulphide and HgCl2 to produce alkyl...
Text Solution
|
- When aniline reacts with NaNO(2) and dil HCl at 0^(@)-5.^(@)C, the pro...
Text Solution
|
- The aniline reaction with…to..yield… as the final product..
Text Solution
|
- Which of the following statements is/are correct?
Text Solution
|
- Which of following statements is/are correct?
Text Solution
|
- Aniline can be obtained by reduction of nitrobenzene with
Text Solution
|