A
B
C
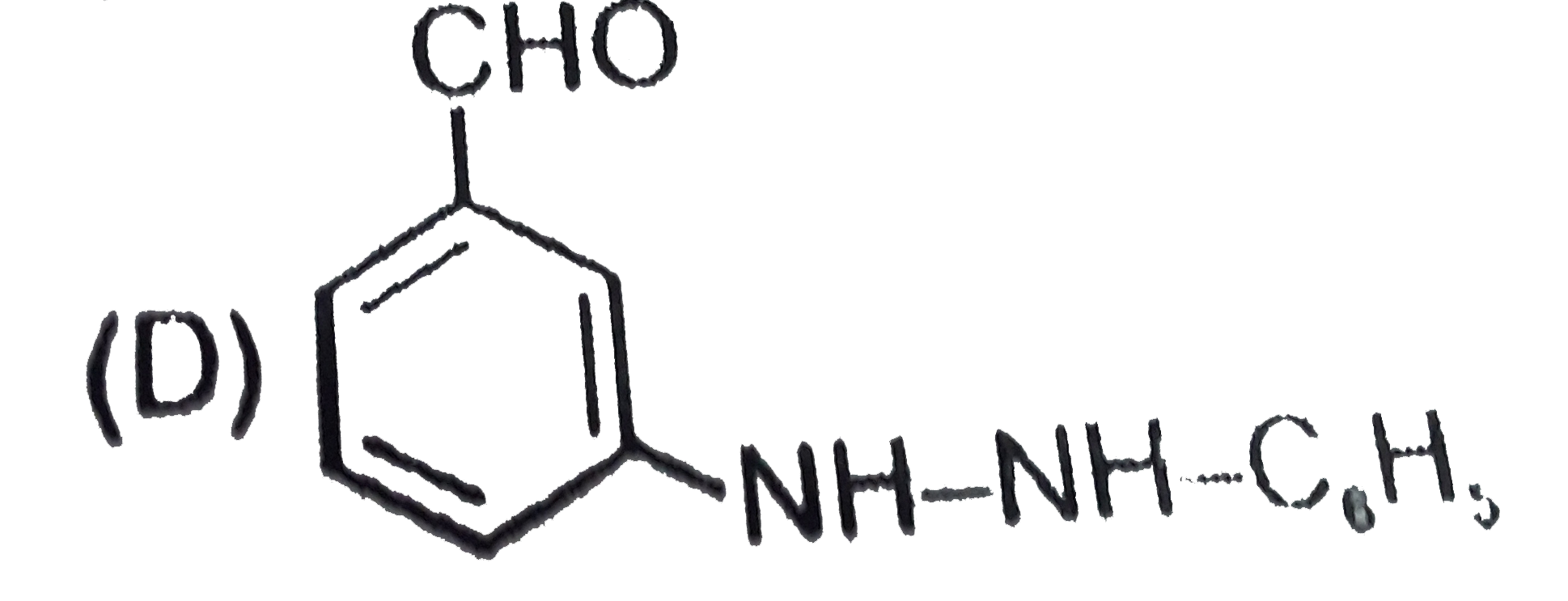
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
AROMATIC COMPOUNDS
RESONANCE ENGLISH|Exercise Section -2 (one or more than one options correct type)|7 VideosAROMATIC COMPOUNDS
RESONANCE ENGLISH|Exercise Section-4: Comprehension type(Only one options correct)|1 VideosAROMATIC COMPOUNDS
RESONANCE ENGLISH|Exercise PART-II: NATIONAL STANDARD EXAMINATION IN CHEMISTRY (NSEC) STAGE-I|31 VideosATOMIC STRUCTURE
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Fundamental Concept )|16 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-AROMATIC COMPOUNDS -PART-III: PRACTICE TEST-2(IIT-JEE(ADVANCED pattern))
- The major product X in the above reaction is
Text Solution
|
- The end product of the following reaction sequence is
Text Solution
|
Text Solution
|
- C(6)H(5)N(2)Cl overset(SnCl(2)//HCl) (A) to overset(C(6)H(5)CHO)to(B) ...
Text Solution
|
- X overset(Sn//HCl) to overset(NaNO(2))underset(HCl)to Z overset(CuCN)u...
Text Solution
|
- Product is:
Text Solution
|
- Which of the following is incorrect:
Text Solution
|