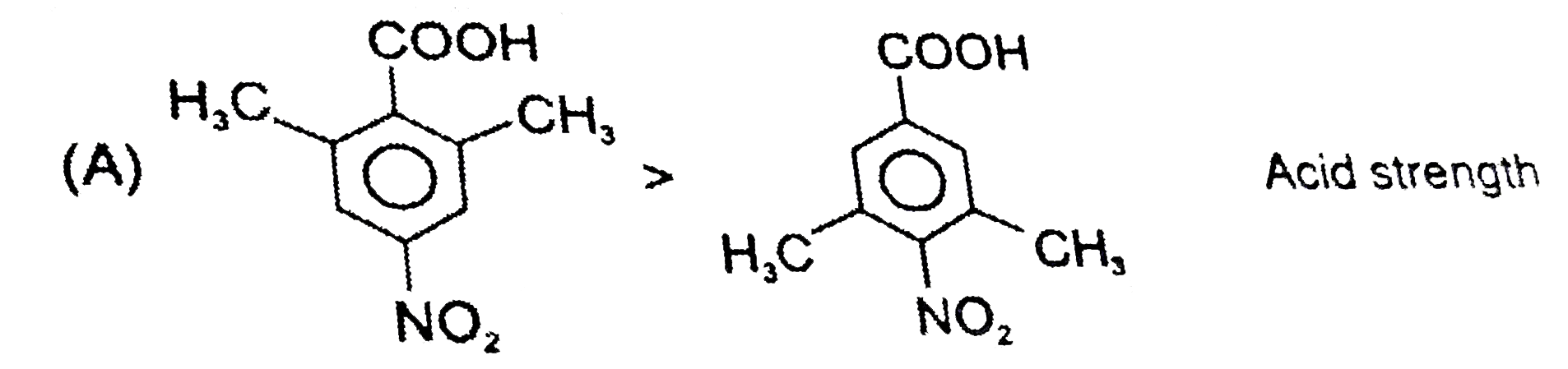
A
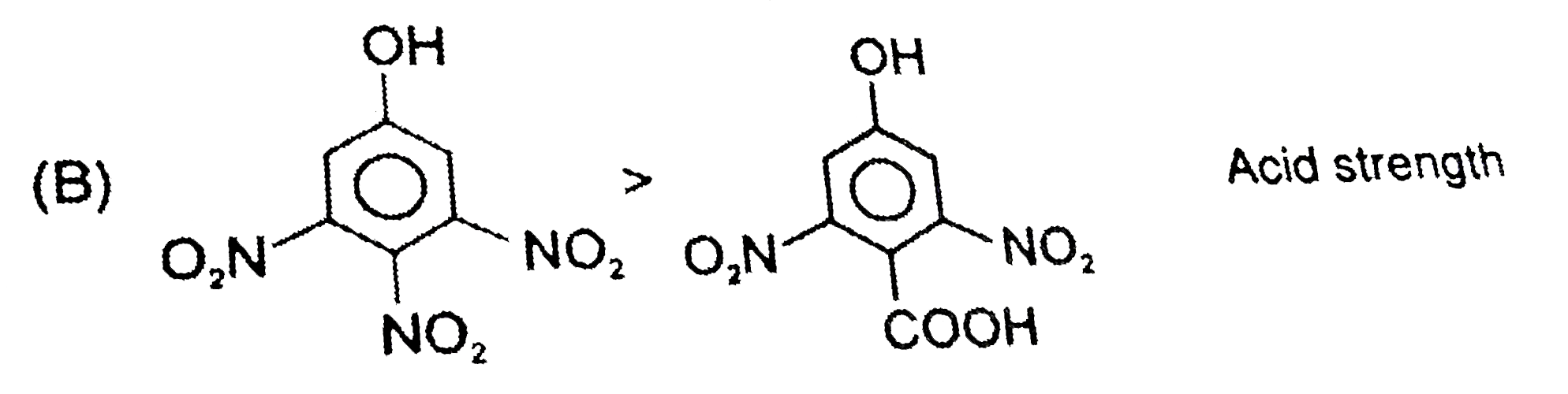
B
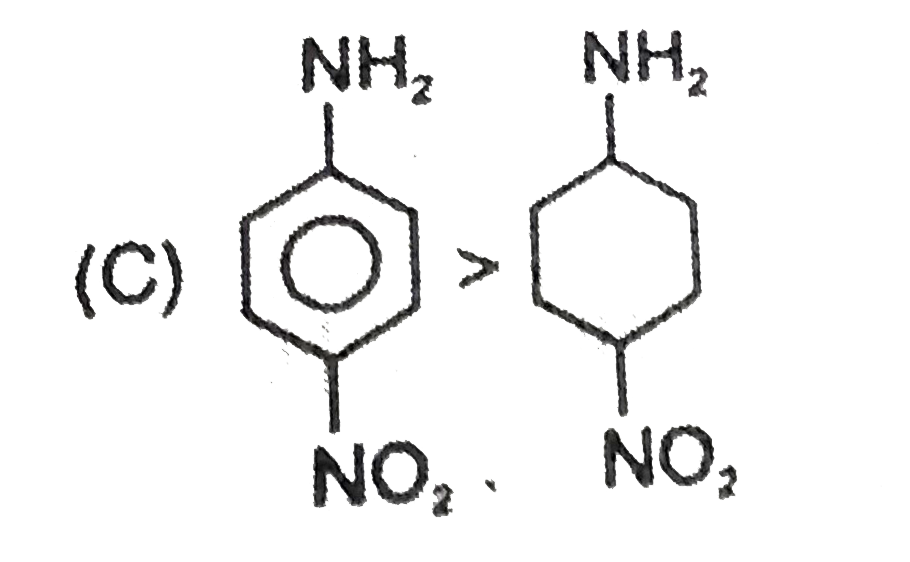
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY II
RESONANCE ENGLISH|Exercise Part-II: Single And Double Value Integer Type|9 VideosGENERAL ORGANIC CHEMISTRY II
RESONANCE ENGLISH|Exercise Part-III: One or More Than One Options Correct Type|9 VideosGENERAL ORGANIC CHEMISTRY II
RESONANCE ENGLISH|Exercise Part-III: Match the Column|2 VideosGASEOUS STATE
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Hydrocarbon)|18 VideosGENERAL ORGANIC CHEMISTRY-I
RESONANCE ENGLISH|Exercise PART-III : PRACTICE TEST-19|1 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-GENERAL ORGANIC CHEMISTRY II-Part-I : Only One Option Correct Type
- Select the most stable intermediates :
Text Solution
|
- Which of the following is most stable carbocation?
Text Solution
|
- The most stable carbocation is:
Text Solution
|
- The following carbocation rearranges to CH(2)=underset(CH(3))underse...
Text Solution
|
- Correct basic strength order is :
Text Solution
|
- The order of basic strength of the given basic nitrogen atoms is :
Text Solution
|
- In the labelled N-atoms which is correct basic strength order:
Text Solution
|
- Choose the strongest base among the following:
Text Solution
|
- Select the basic strength order of following molecules ?
Text Solution
|
- Which is the weakest base among the followings ?
Text Solution
|
- Write the order of K(a(1)) values of following acids :
Text Solution
|
- The acid strength order is :
Text Solution
|
- (X) (C(6)H(3)CIBrCOOH) are a dihalosubstituted benzoic acids. The stro...
Text Solution
|
- The order of acidity of the H-atoms underlined in the following compou...
Text Solution
|
- Most acidic hydrogen is present in
Text Solution
|
- The correct orders are:
Text Solution
|
- Observe the following sequence of reactions : Select the correct ...
Text Solution
|
- Order of K(a) which can be predicted by following reaction is:
Text Solution
|
- The gases produced in the following reactions are respectively I: CH...
Text Solution
|
- Decreasing order of enol content of the following compounds in liquid ...
Text Solution
|
 basicity order
basicity order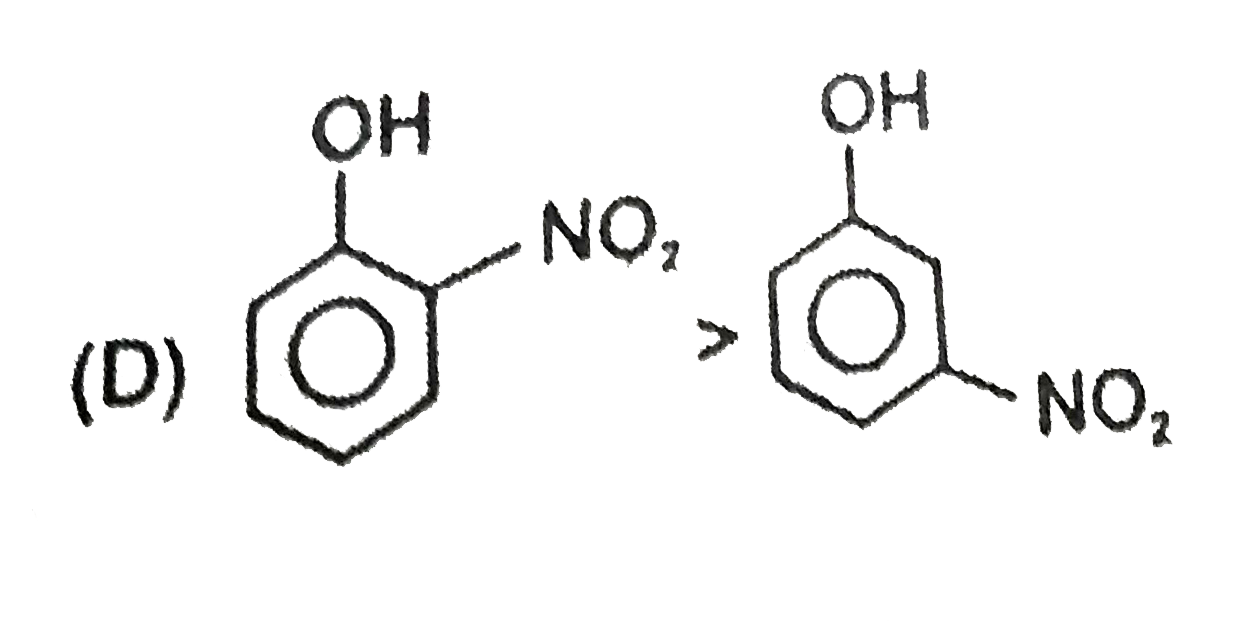 acidity order
acidity order