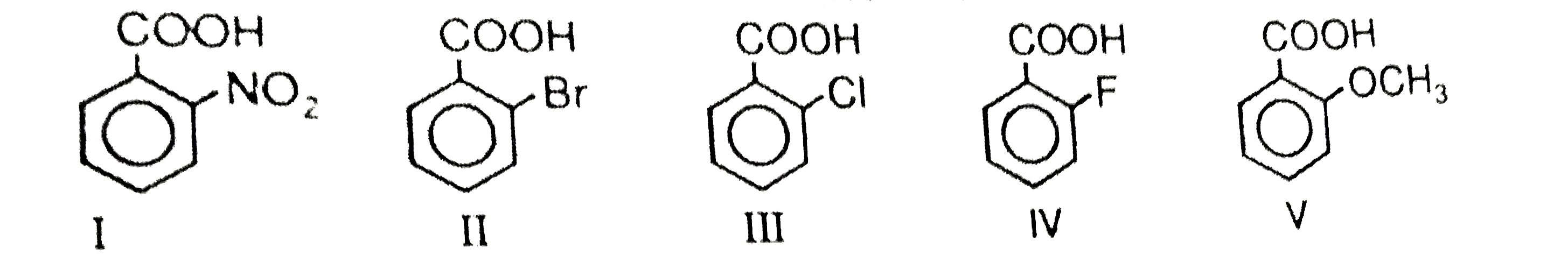
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY II
RESONANCE ENGLISH|Exercise Exercise-3 Part:I JEE(Advanced)/IIT-JEE Problems (Previous Years)|17 VideosGENERAL ORGANIC CHEMISTRY II
RESONANCE ENGLISH|Exercise Exercise-3 Part:II JEE(MAIN)/AIEEE PROBLEMS (PREVIOUS YEARS)|18 VideosGENERAL ORGANIC CHEMISTRY II
RESONANCE ENGLISH|Exercise Part-III: One or More Than One Options Correct Type|9 VideosGASEOUS STATE
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Hydrocarbon)|18 VideosGENERAL ORGANIC CHEMISTRY-I
RESONANCE ENGLISH|Exercise PART-III : PRACTICE TEST-19|1 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-GENERAL ORGANIC CHEMISTRY II-Part-IV : Comprehension
- Reaction intermdiates are short lived species and are highly reactive....
Text Solution
|
- Reaction intermdiates are short lived species and are highly reactive....
Text Solution
|
- Reaction intermdiates are short lived species and are highly reactive....
Text Solution
|
- Ortho effect is special type of effect that is shown by o-subsituents ...
Text Solution
|
- Ortho effect is special type of effect that is shown by o-subsituents ...
Text Solution
|
- Ortho effect is special type of effect that is shown by o-subsituents ...
Text Solution
|
- The lone pair of amines makes them basic. They react with acids to for...
Text Solution
|
- The lone pair of amines makes them basic. They react with acids to for...
Text Solution
|
- The lone pair of amines makes them basic. They react with acids to for...
Text Solution
|
- Observe the following reaction and answer the following questions : ...
Text Solution
|
- Observe the following reaction and answer the following questions : ...
Text Solution
|
- Answer questions 1, 2 and 3 by appropriately matching the information ...
Text Solution
|