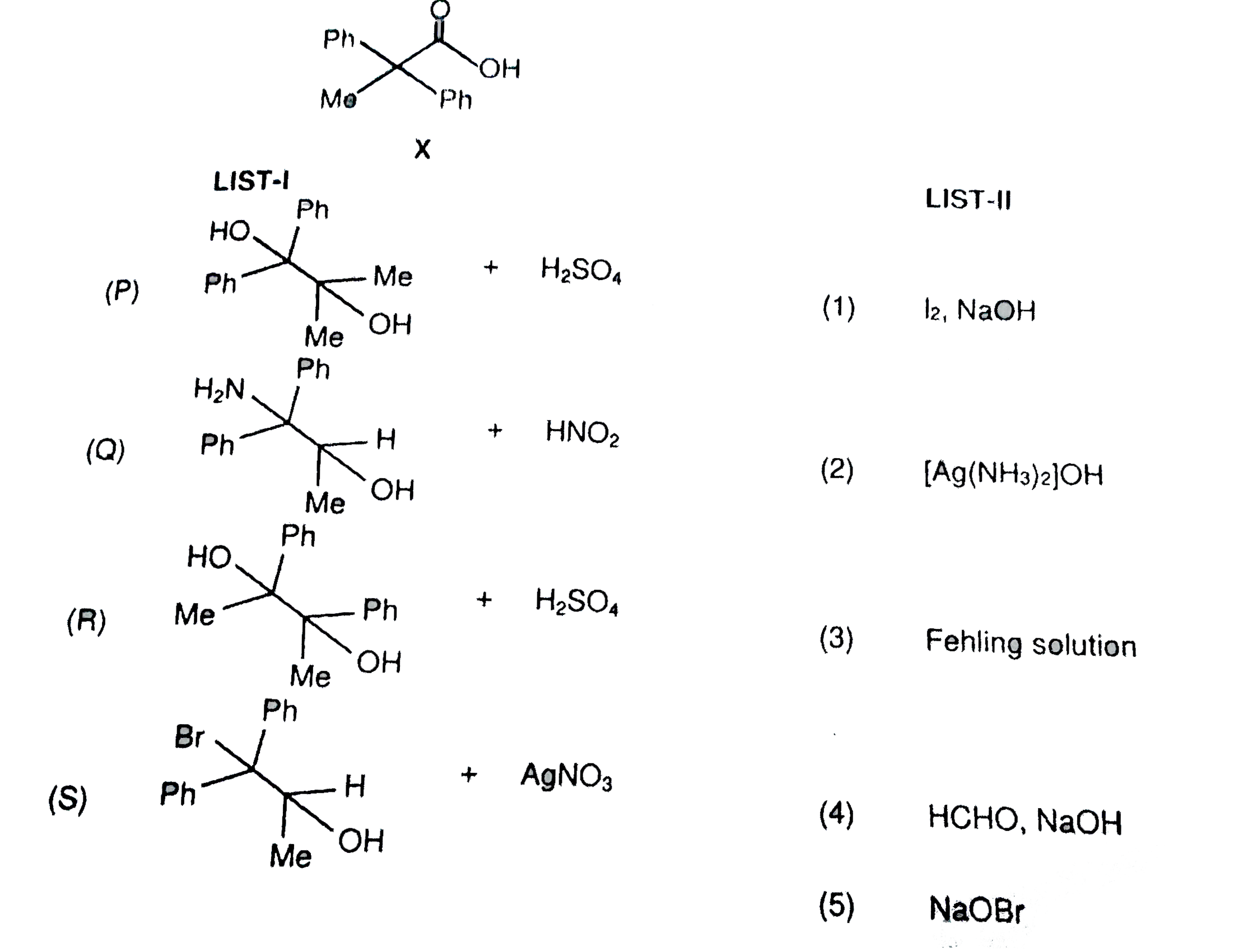A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ORGANIC REACTION MECHANISMS-IV
RESONANCE ENGLISH|Exercise Exercise-3 Part-2 JEE (MAIN ) OFFLINE PROBLEMS|10 VideosORGANIC REACTION MECHANISMS-IV
RESONANCE ENGLISH|Exercise Exercise-3 Part-2 JEE (MAIN ) ONLINE PROBLEMS|10 VideosORGANIC REACTION MECHANISMS-IV
RESONANCE ENGLISH|Exercise Exercise-2 Part-4|8 VideosORGANIC REACTION MECHANISMS - II
RESONANCE ENGLISH|Exercise APSP Part - 3|22 VideosPERIODIC TABLE & PERIODICITY
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(BASIC CONCEPTS)|27 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-ORGANIC REACTION MECHANISMS-IV-Exercise-3 Part-1
- Identify (X),(Y) and (Z) in the following synthetic scheme and write t...
Text Solution
|
- Identify the set of reagents/ reaction condition ‘X’ and ‘Y’ in the fo...
Text Solution
|
- How many structures of F is possible?
Text Solution
|
- Which of the reagents on reaction with cyclohexanol gives best yield o...
Text Solution
|
- Match the following (one term in column-I may match with more than one...
Text Solution
|
- In the following reaction sequence , product I,J and L are formed , K ...
Text Solution
|
- In the following sequence, product I, ,J and L are formed. K represent...
Text Solution
|
- In the following sequence, product I,J and L are formedK represents a ...
Text Solution
|
- The total number of alkenes possible by dehydrobromination of 3-bromo-...
Text Solution
|
- An acyclic hydrocarbon P, having molecular fromula C(6)H(10) gave acet...
Text Solution
|
- An acyclic hydrocarbon P, having molecular formula C6H10 , gave aceton...
Text Solution
|
- The major product (H) in the given reaction sequence is : CH3-CH2-CO...
Text Solution
|
- Match the chemical conversion in List-I with the approprotae reagents ...
Text Solution
|
- In the following reactions : Compound X is
Text Solution
|
- In the following reactoins The major compound Y is
Text Solution
|
- The number of hydroxyl group(s) in Q is overset(H^(+))underset("hea...
Text Solution
|
- The desired product X can be prepared by reacting the major product of...
Text Solution
|
- LIST-I contains reactions and LIST-II contains major products.
Text Solution
|