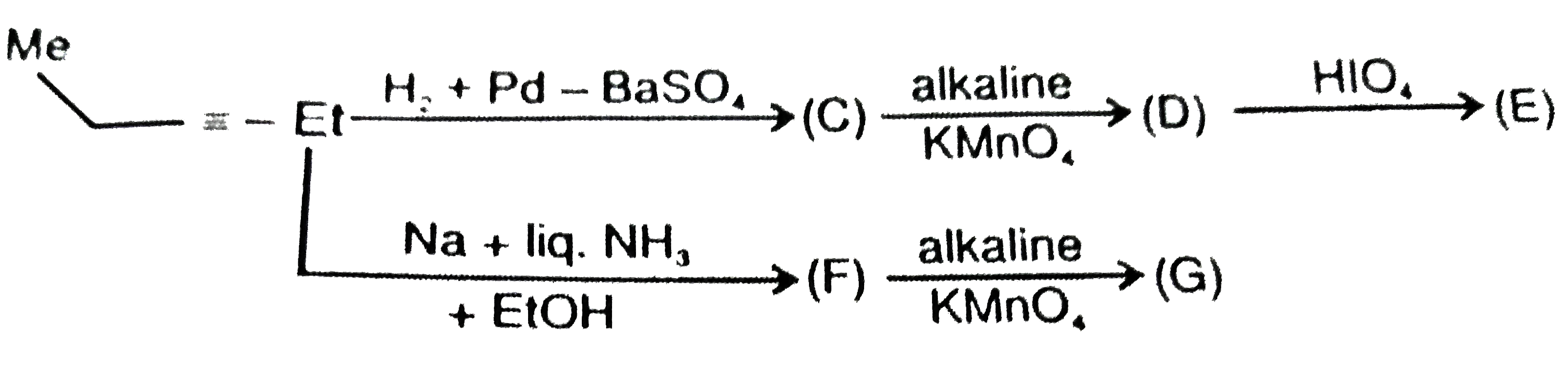
A
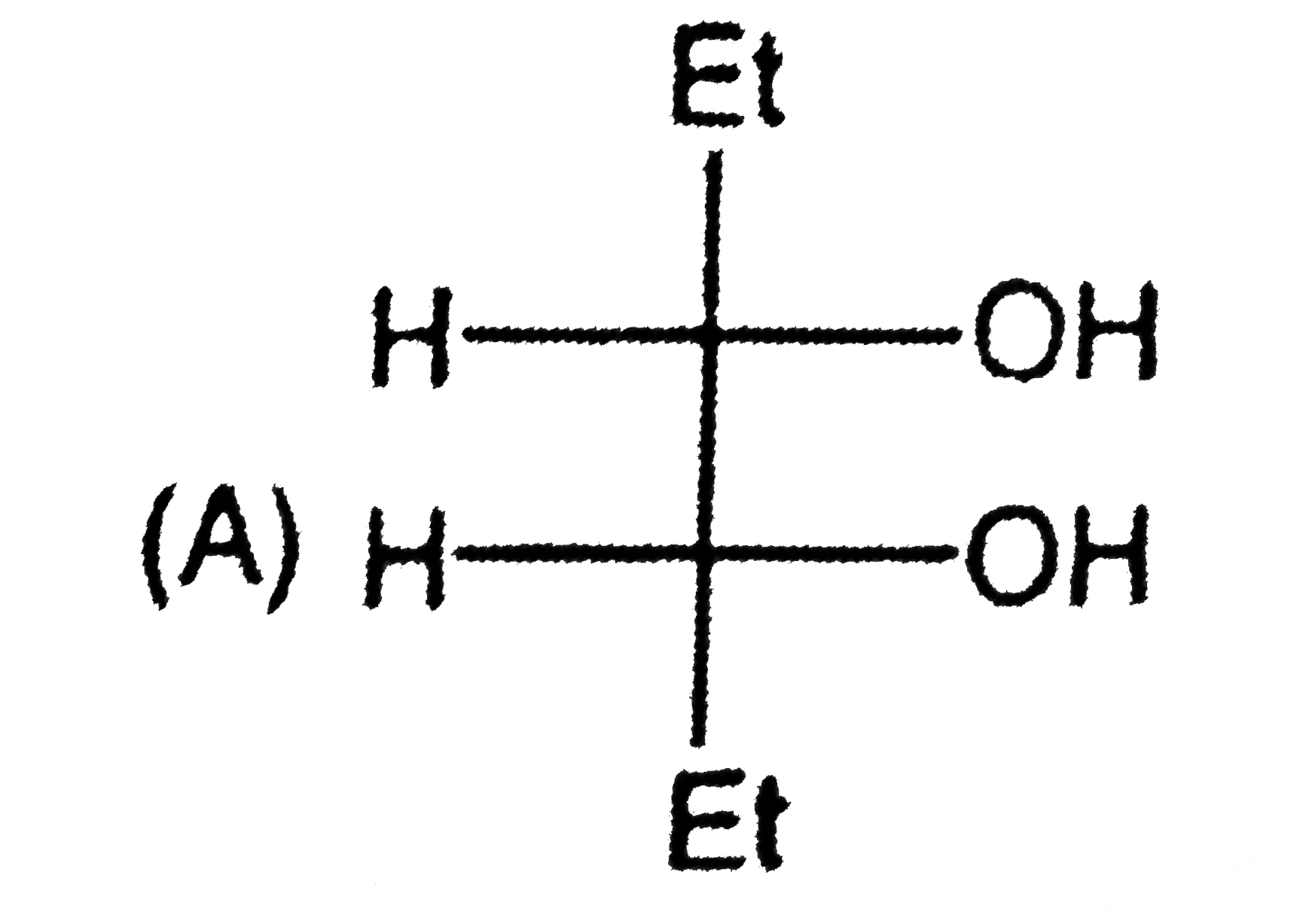
B
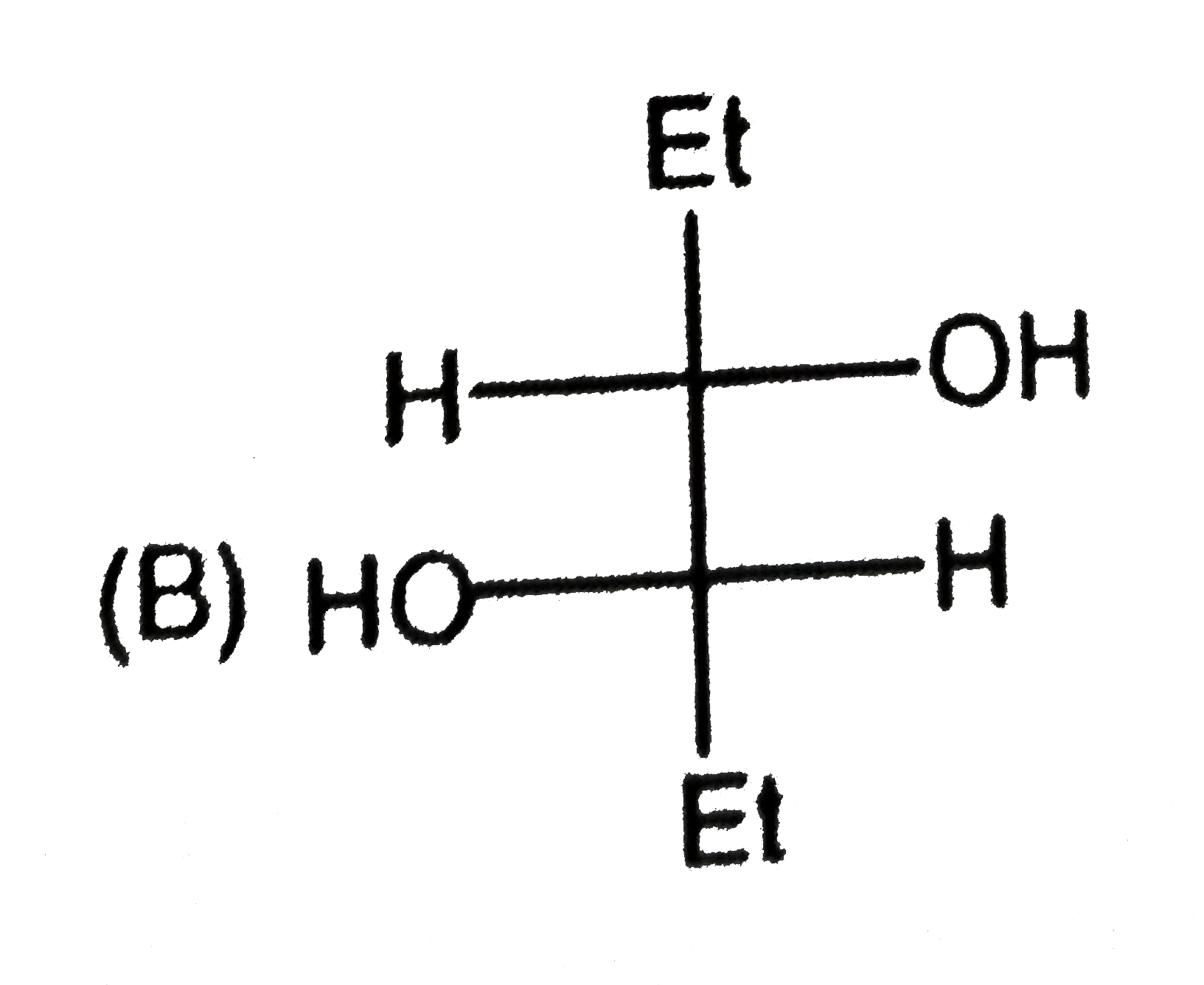
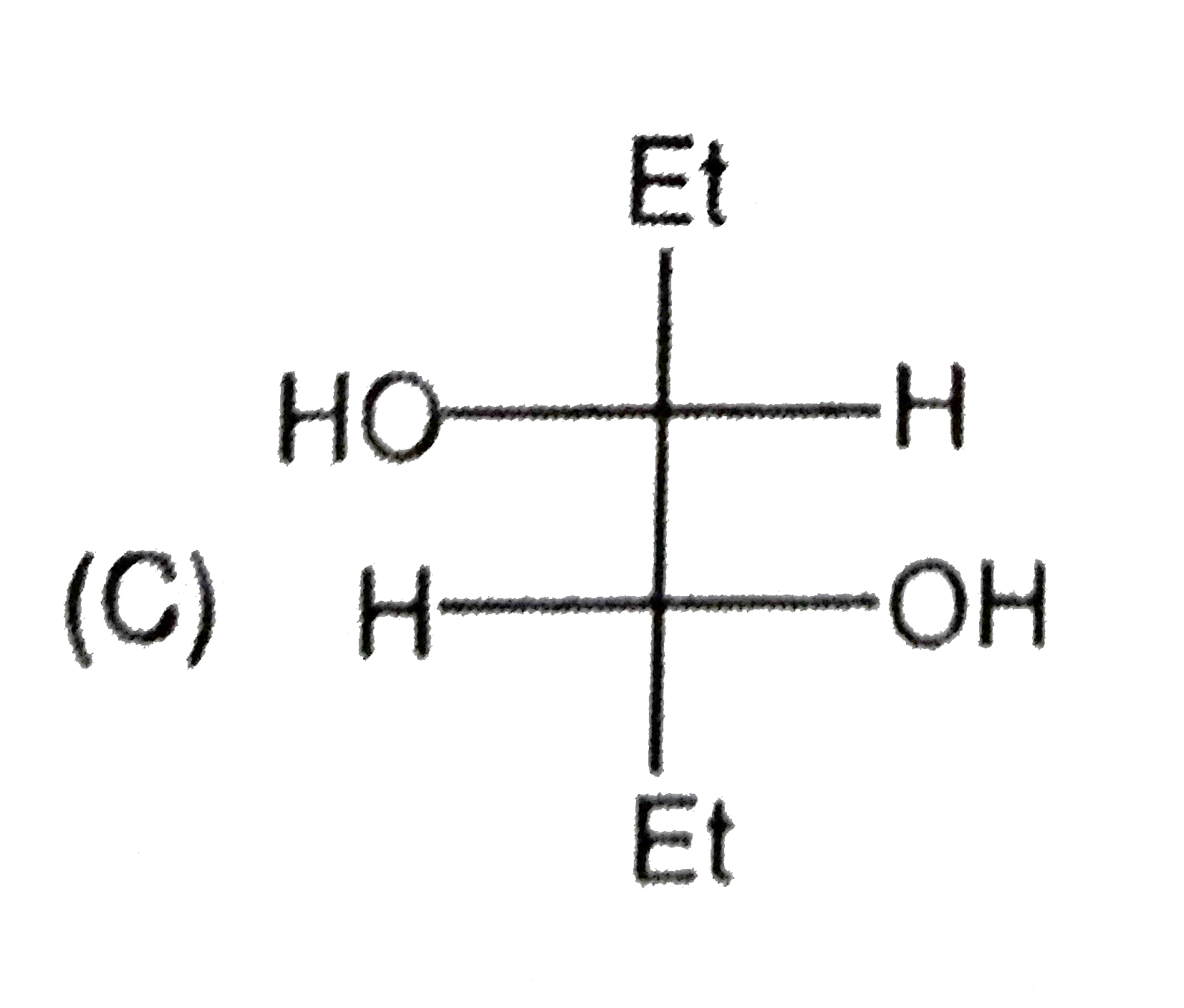
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDUCTION, OXIDATION & HYDROLYSIS REACTIONS
RESONANCE ENGLISH|Exercise Part-I : Jee (Advanced) (Previous years)|12 VideosREDUCTION, OXIDATION & HYDROLYSIS REACTIONS
RESONANCE ENGLISH|Exercise Part-II JEE (Main) AIEEE Problems (Previous Years)|16 VideosREDUCTION, OXIDATION & HYDROLYSIS REACTIONS
RESONANCE ENGLISH|Exercise Part-III: One or more than one options correct type|4 VideosPERIODIC TABLE & PERIODICITY
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(BASIC CONCEPTS)|27 VideosS BLOCK ELEMENTS
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Biomolecules & Polymer)|4 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-REDUCTION, OXIDATION & HYDROLYSIS REACTIONS-Part-IV: Comprehension
- (P) and (Q) respectively are
Text Solution
|
- (R) is:
Text Solution
|
- Oxidation state exhibited by Mn in KMnO(4) is
Text Solution
|
- Oxidation state exhibited by Mn in MnO(2) is
Text Solution
|
- Oxidation state exhibited by Mn in MnO(4)^(2-) is
Text Solution
|
- The compound (G) is :
Text Solution
|
- Oxidation state exhibited by Cr in K(2)CrO(4) is
Text Solution
|
- Oxidation state exhibited by S in H(2)S is
Text Solution
|
- The only correct combination in which product gives positive test with...
Text Solution
|
- Oxidation state exhibited by Fe in Fe(CO)(5) is
Text Solution
|