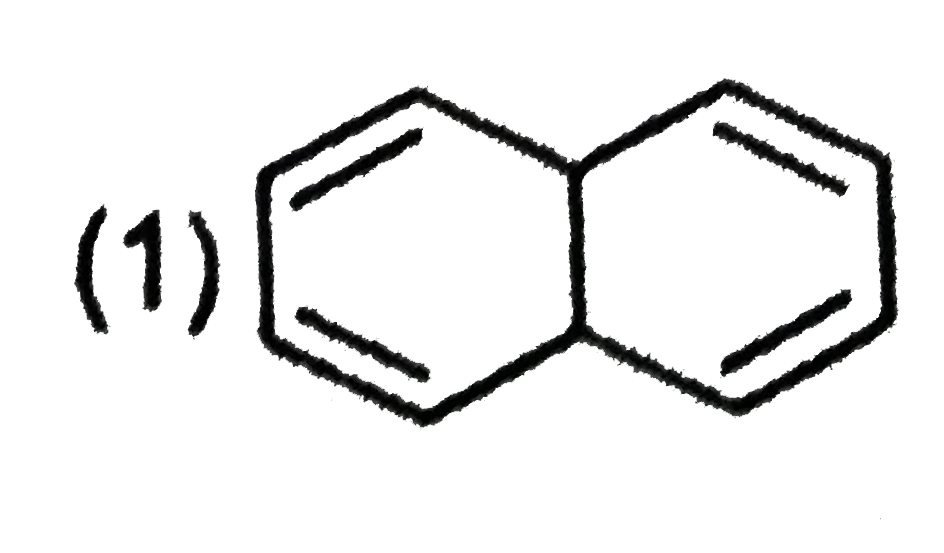
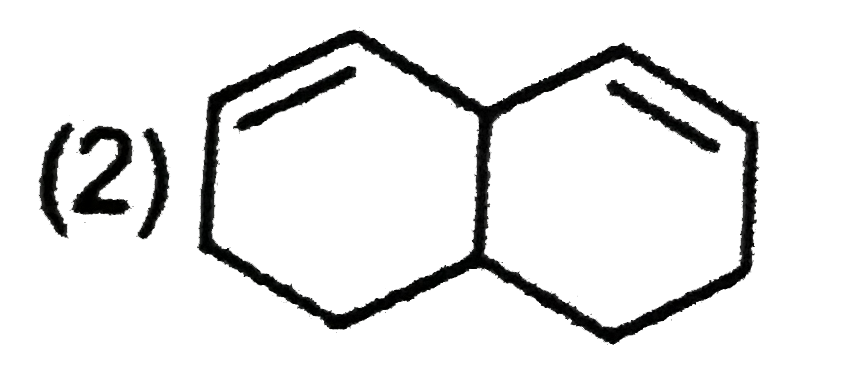
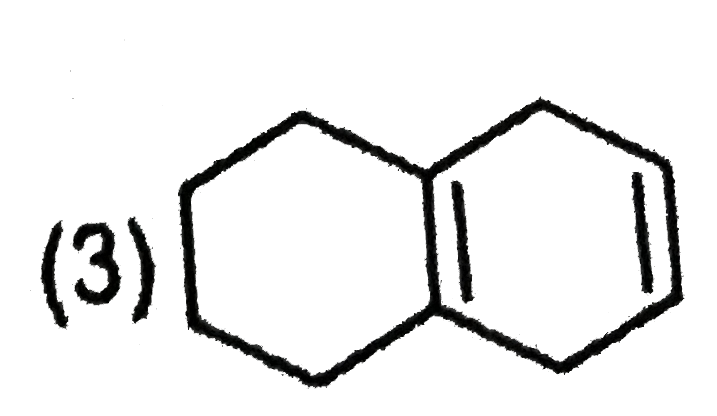
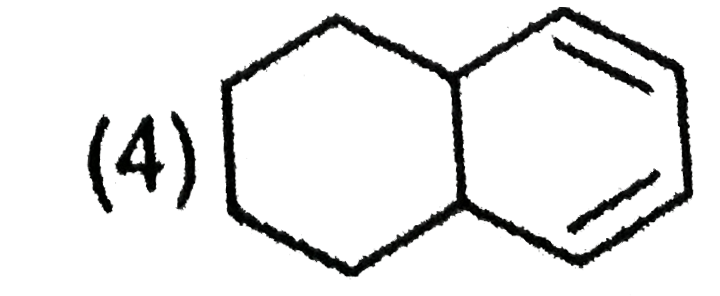
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
REDUCTION, OXIDATION & HYDROLYSIS REACTIONS
RESONANCE ENGLISH|Exercise Part-II : National Standard Examination in Chemistry|27 VideosREDUCTION, OXIDATION & HYDROLYSIS REACTIONS
RESONANCE ENGLISH|Exercise Part-III : High Level Problems (Subjective Questions)|6 VideosREDUCTION, OXIDATION & HYDROLYSIS REACTIONS
RESONANCE ENGLISH|Exercise JEE (MAIN) ONLINE PROBLEMS|13 VideosPERIODIC TABLE & PERIODICITY
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(BASIC CONCEPTS)|27 VideosS BLOCK ELEMENTS
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Biomolecules & Polymer)|4 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-REDUCTION, OXIDATION & HYDROLYSIS REACTIONS-Additional problems for self Practice (Part-1)
- What are the oxidation states of chromium in the following : CrO(4)^-...
Text Solution
|
- An alkene on ozonolysis yields only ethanal. There is an isomer of the...
Text Solution
|
- Chromium chloride reacts with sodium hydroxide to form a green precipi...
Text Solution
|
- Which of the following will declourise alkaline KMnO(4)solution ?
Text Solution
|
- Baeyer's reagent is
Text Solution
|
- Ferric sulphate reacts with sodium hydroxide to form a reddish brown p...
Text Solution
|
- , Product B and C are respectively :
Text Solution
|
- Complete the following reaction
Text Solution
|
- Fenton's reagent is :
Text Solution
|
- The reagent with which both acetaldehyde and acetone react easily, is ...
Text Solution
|
- Heating of sodium acetate with soda lime produces
Text Solution
|
- When acetaldehyde is heated with Fehling's solution, it given a precip...
Text Solution
|
- Heating of PCl(5) with formic acid produces
Text Solution
|
- Which of the following reaction involves homogeneous reduction ?
Text Solution
|
- X is:
Text Solution
|
- Which reducing agent, you can't use to carry out the following transfo...
Text Solution
|
- Complete the following reaction
Text Solution
|
- An unknown compound decolorizes bromine in carbon tetrachloride, and i...
Text Solution
|
- The reaction, Ph-CH(2)-CH=CH-overset(OH)overset(|)(CH)-CH(3) underset(...
Text Solution
|
- The reagent used to convert RCOOH rarr RCH(2)OH is
Text Solution
|