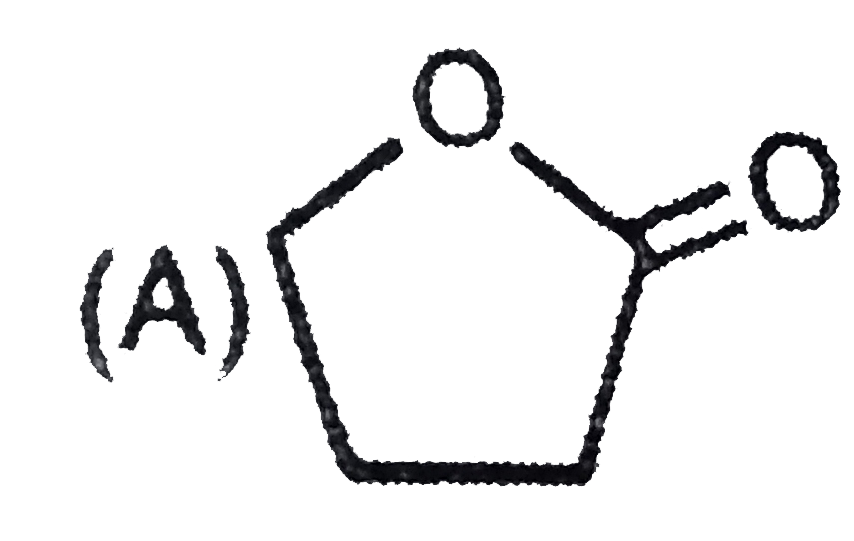
A
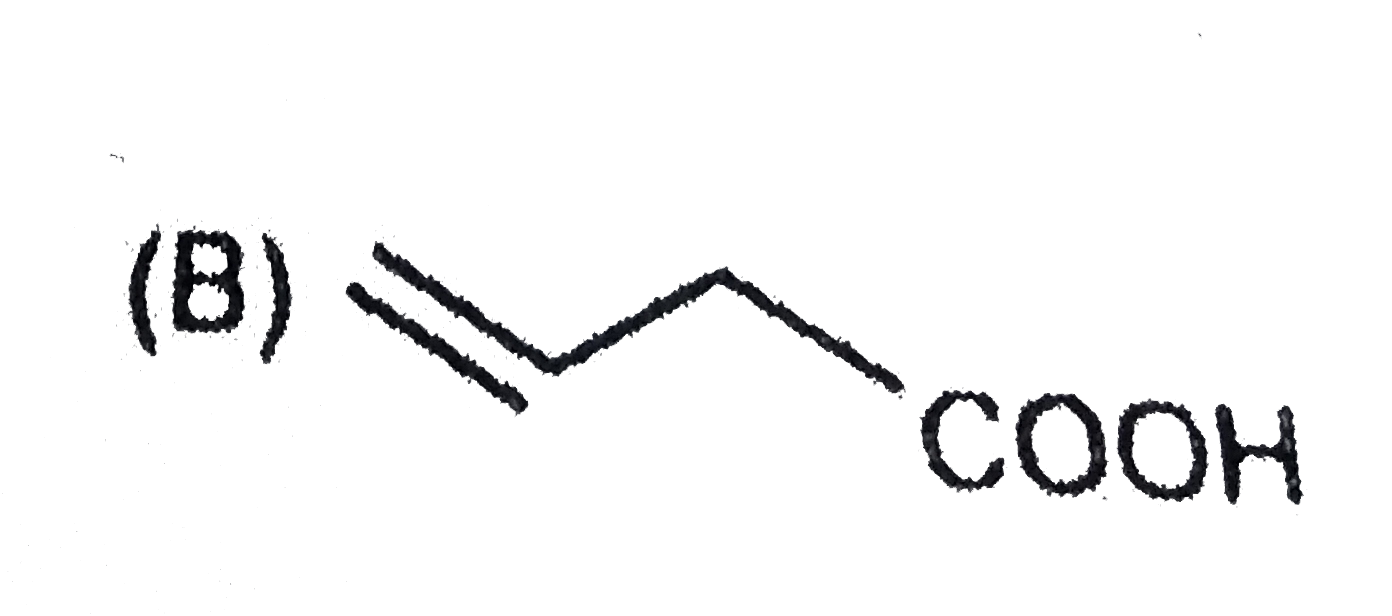
B
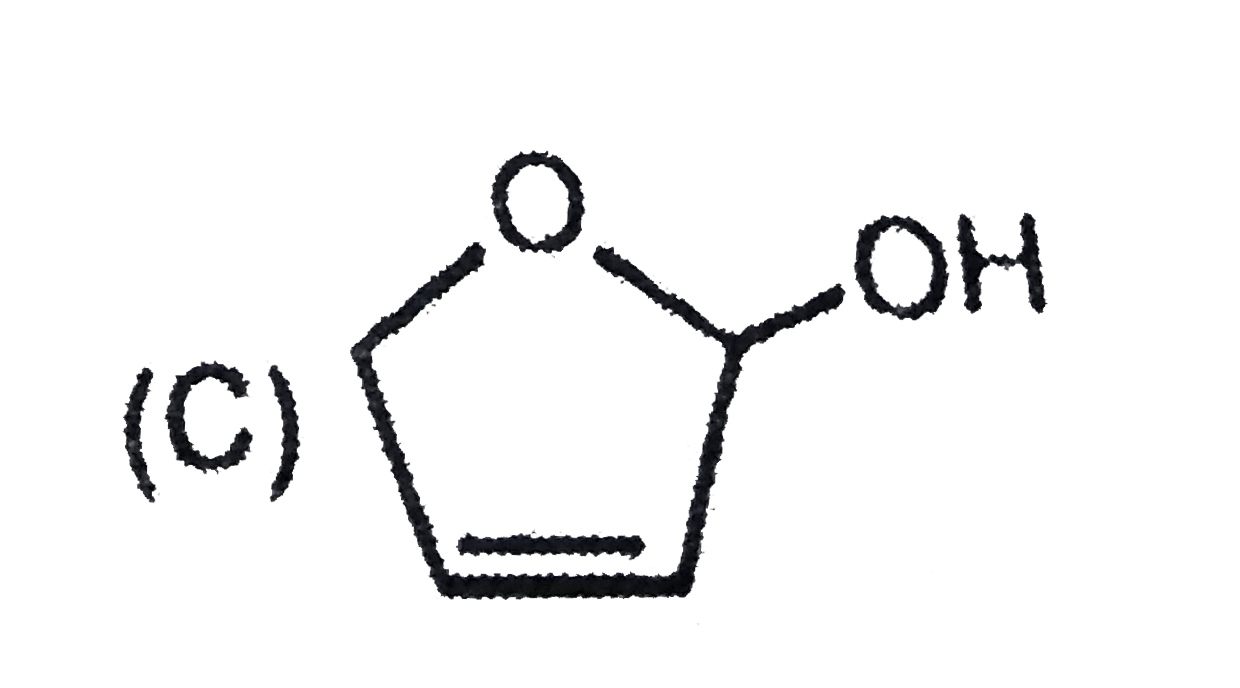
C
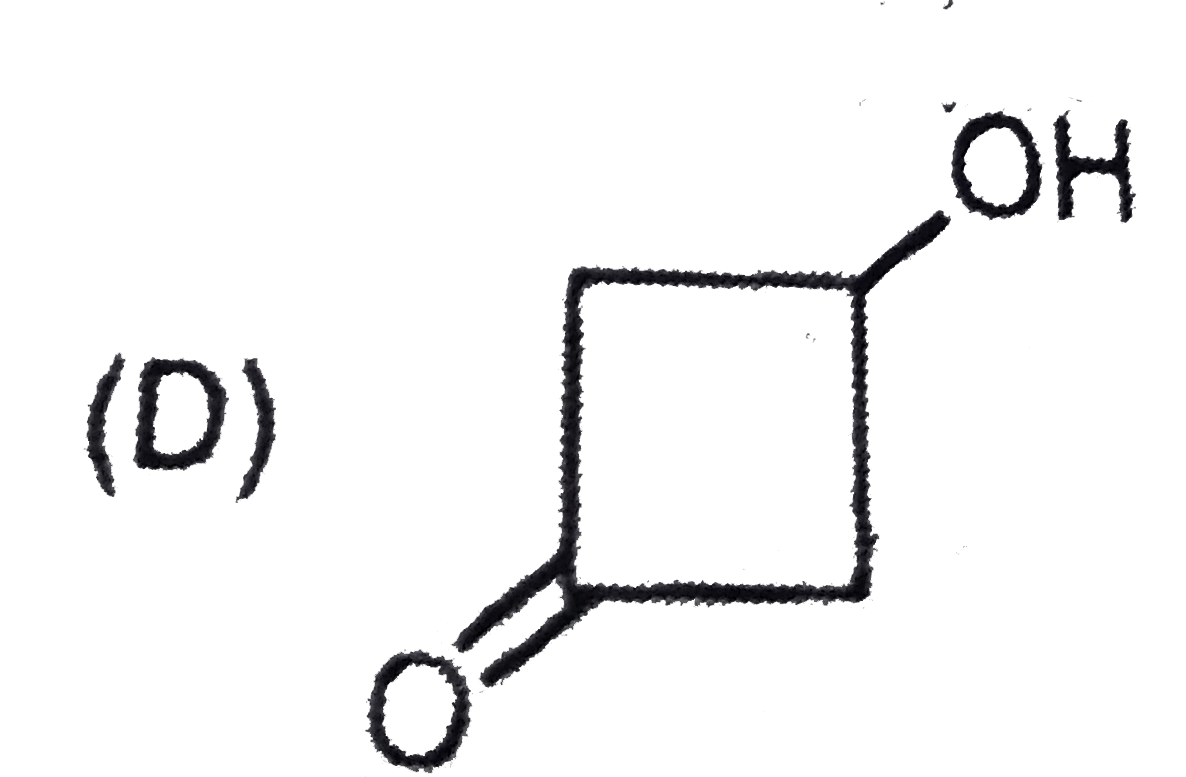
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
REDUCTION, OXIDATION & HYDROLYSIS REACTIONS
RESONANCE ENGLISH|Exercise Part-III : High Level Problems (Subjective Questions)|6 VideosREDUCTION, OXIDATION & HYDROLYSIS REACTIONS
RESONANCE ENGLISH|Exercise Only One Option Correct Type|4 VideosREDUCTION, OXIDATION & HYDROLYSIS REACTIONS
RESONANCE ENGLISH|Exercise Additional problems for self Practice (Part-1)|30 VideosPERIODIC TABLE & PERIODICITY
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(BASIC CONCEPTS)|27 VideosS BLOCK ELEMENTS
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Biomolecules & Polymer)|4 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-REDUCTION, OXIDATION & HYDROLYSIS REACTIONS-Part-II : National Standard Examination in Chemistry
- The reaction, R(2)CO+4[H] underset("Conc. HCl")overset("Zn-Hg")rarr R(...
Text Solution
|
- Acetone will be obtained on ozonolysis of
Text Solution
|
- Which of the following are reducing agents among the following?
Text Solution
|
- Reduction of an isonitrile gives a
Text Solution
|
- Methane may be obtained from monochloromethane by
Text Solution
|
- The compound which does not react with lithium aluminium hydride is
Text Solution
|
- The compound which would yield 5- Oxo-2-methylhexanal on reductive ozo...
Text Solution
|
- Reduction of methylbenzoate (C(6)H(5)COOCH(3)) to benzyl alcohol (C(6)...
Text Solution
|
- Oxidation of cyclopentanol to cyclopentanone can be accomplished by us...
Text Solution
|
- Carbonyl compounds can generally be converted to hydrocarbons by
Text Solution
|
- Which of the following reagents would not be a good choice for reducin...
Text Solution
|
- Suggest the suitable reagent for the following transformation.
Text Solution
|
- An isocyanide on reduction with hydrogen in the presence of platinum g...
Text Solution
|
- Compound X (C(5)H(10)O) is a chiral alcohol. It is catalytically hydro...
Text Solution
|
- 4-Oxobutanoic acid is reduced with Na-borohydride and the product is t...
Text Solution
|
- A solution of sodium metal in liquid ammonia is strongly reducing due ...
Text Solution
|
- Which of the following statements is true for the reaction given below...
Text Solution
|
- Complete catalytic hydrogenation of napthalene gives decalin (C(10)H(1...
Text Solution
|
- Which of the following on treatment with hot concentrated acidified KM...
Text Solution
|
- The correct sequence of reagents from those listed below for the follo...
Text Solution
|