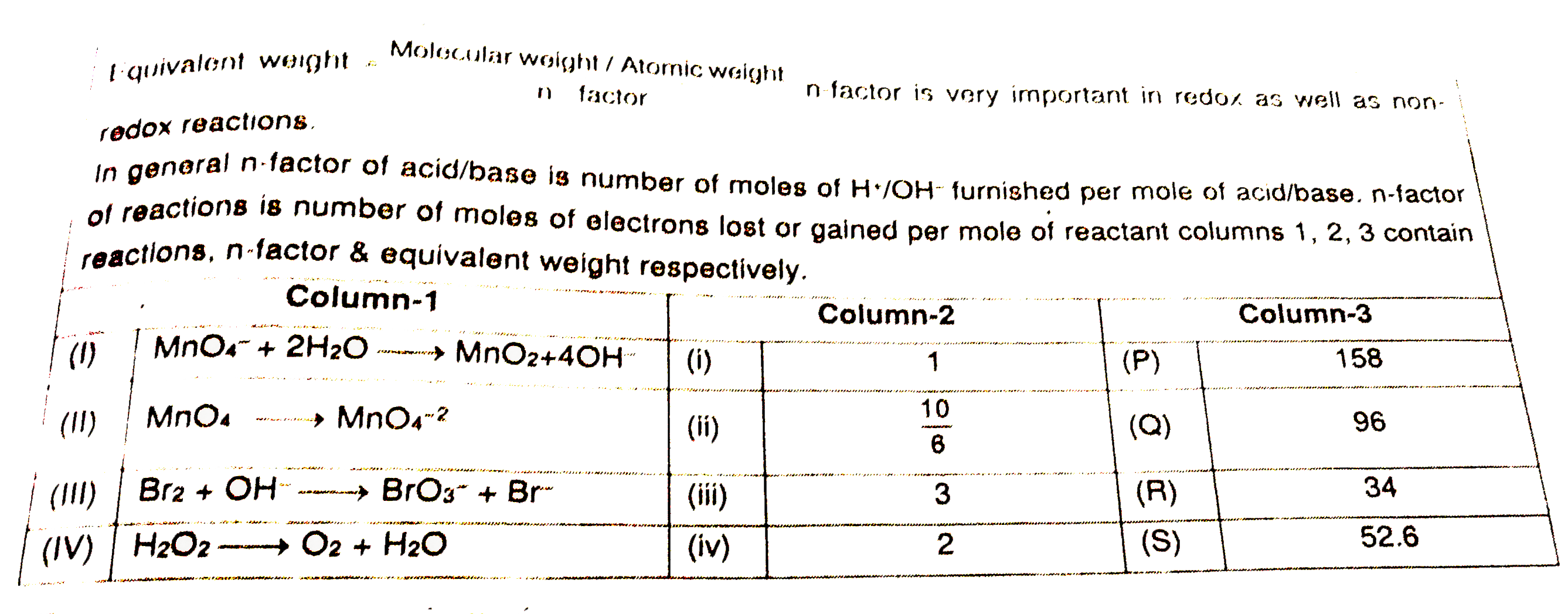
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
EQUIVALENT CONCEPT & TITRATIONS
RESONANCE ENGLISH|Exercise Exercise -3 (Part-I)|5 VideosEQUIVALENT CONCEPT & TITRATIONS
RESONANCE ENGLISH|Exercise Exercise -3 (Part-II)|7 VideosEQUIVALENT CONCEPT & TITRATIONS
RESONANCE ENGLISH|Exercise Exercise -2 (Part-III)|5 VideosELECTROCHEMISRY
RESONANCE ENGLISH|Exercise Advanced Level Problems|88 VideosFUNDAMENTAL CONCEPT
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Fundamental Concept )|40 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-EQUIVALENT CONCEPT & TITRATIONS-Exercise -2 (Part-IV)
- Equivalent mass of Ba(MnO(4))(2) in acidic medium is :(where M stands ...
Text Solution
|
- Calculate the equivalent weight of chlorine in the reaction of Chlorin...
Text Solution
|
- Equivalent weight of oxalic acid salt in following reaction is :( Atom...
Text Solution
|
- Calculate the normality of 18 volume hydrogen peroxide ...
Text Solution
|
- Some amount of "20V" H(2)O(2) is mixed with excess of acidified soluti...
Text Solution
|
- Hydride of Be is Covalent while that of Na is ionic Why ?
Text Solution
|
- For KMnO(4) in neutral medium correct combination is -
Text Solution
|
- For KMnO(4) in neutral medium correct combination is -
Text Solution
|
- Hg2Cl2 + NH4OH to
Text Solution
|