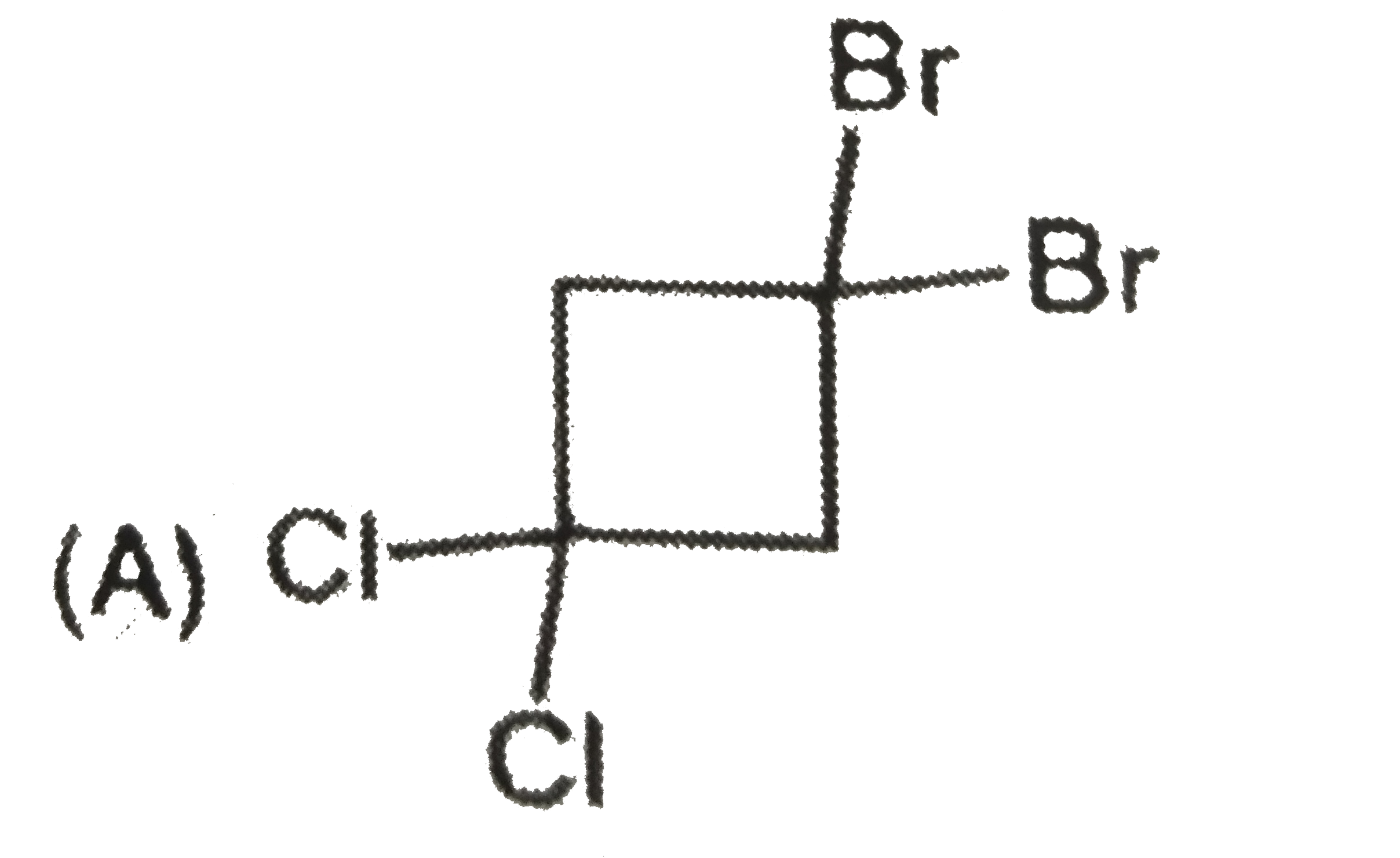
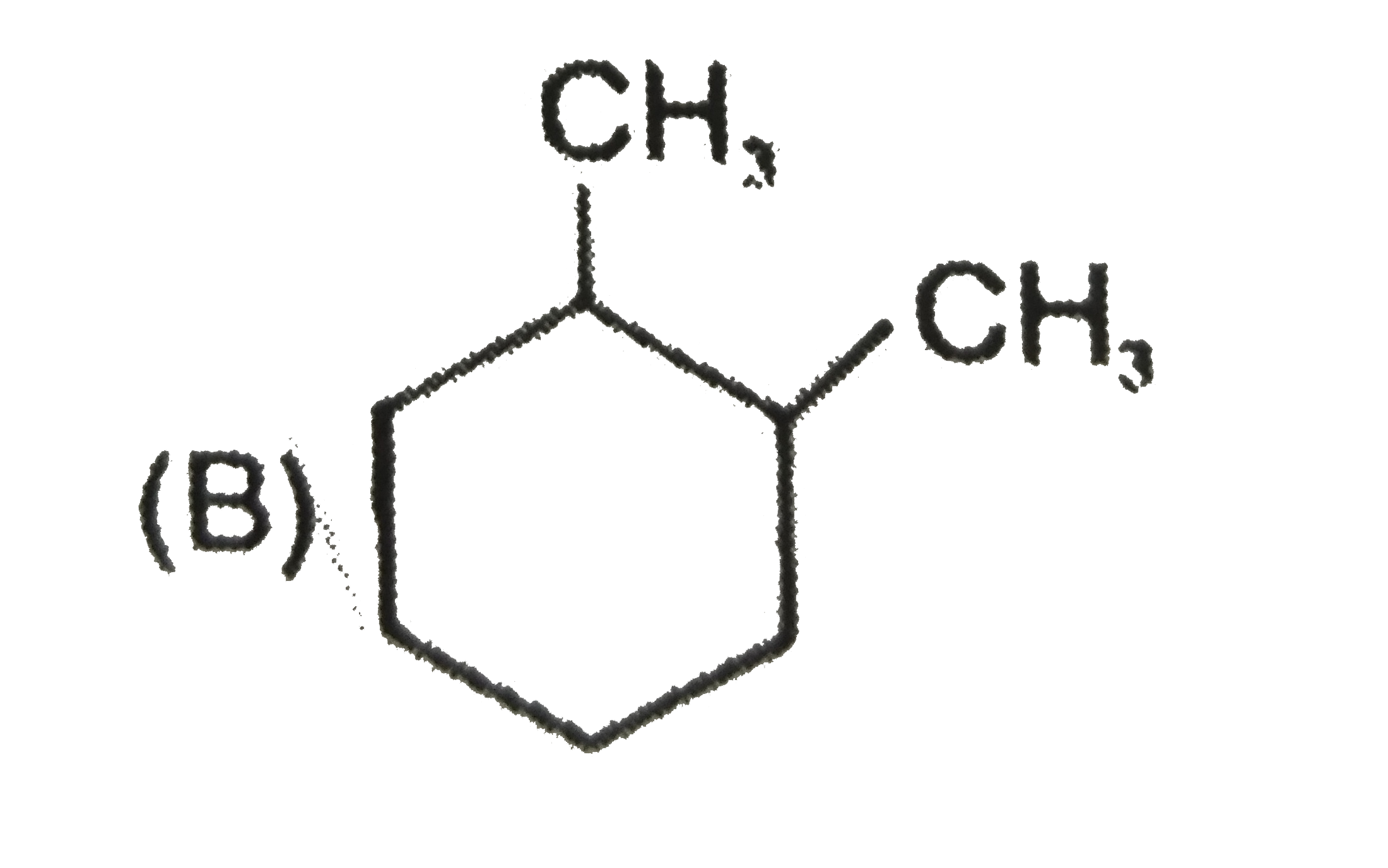
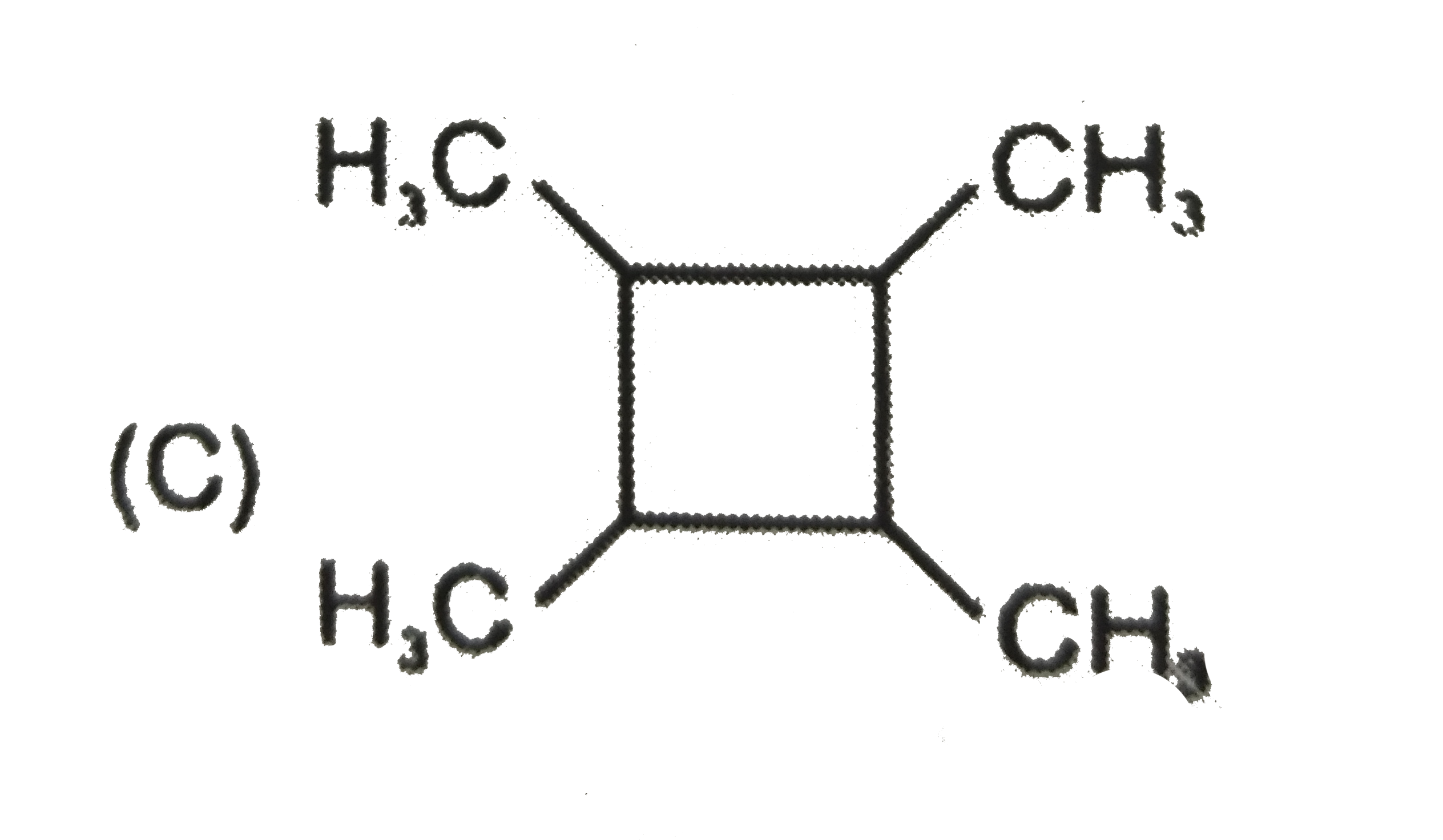
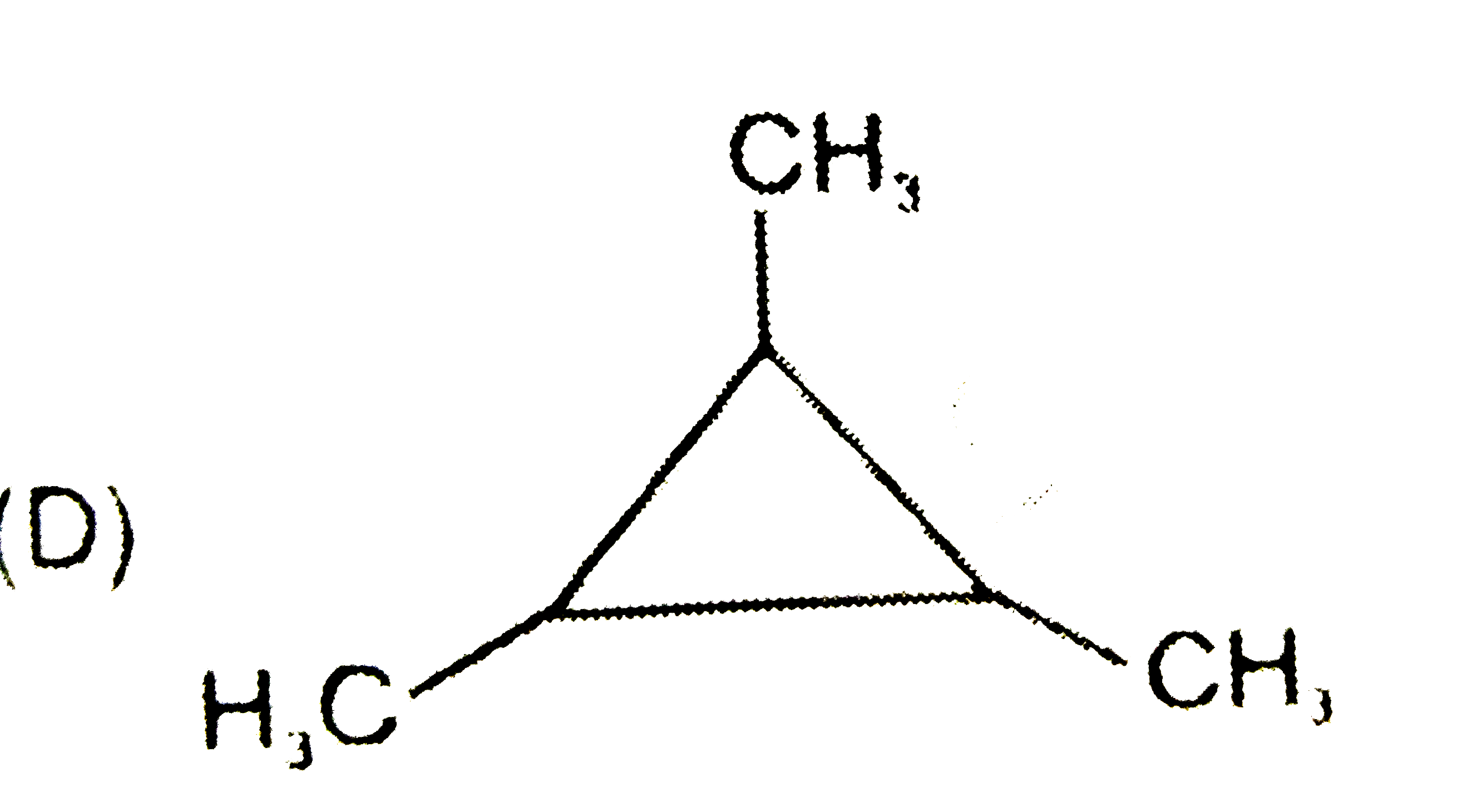
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
STEREOISOMERISM
RESONANCE ENGLISH|Exercise EXERCISE (PART II SECTION (B))|3 VideosSTEREOISOMERISM
RESONANCE ENGLISH|Exercise EXERCISE (PART II SECTION (C))|3 VideosSTEREOISOMERISM
RESONANCE ENGLISH|Exercise EXERCISE (PART 1 SECTION (K))|2 VideosSOLUTIONS
RESONANCE ENGLISH|Exercise Advabced Level Problems (PART-2)|35 VideosSTRUCTURAL IDENTIFICATION
RESONANCE ENGLISH|Exercise Advanced level Problems (Part-III)|12 Videos
Similar Questions
Explore conceptually related problems