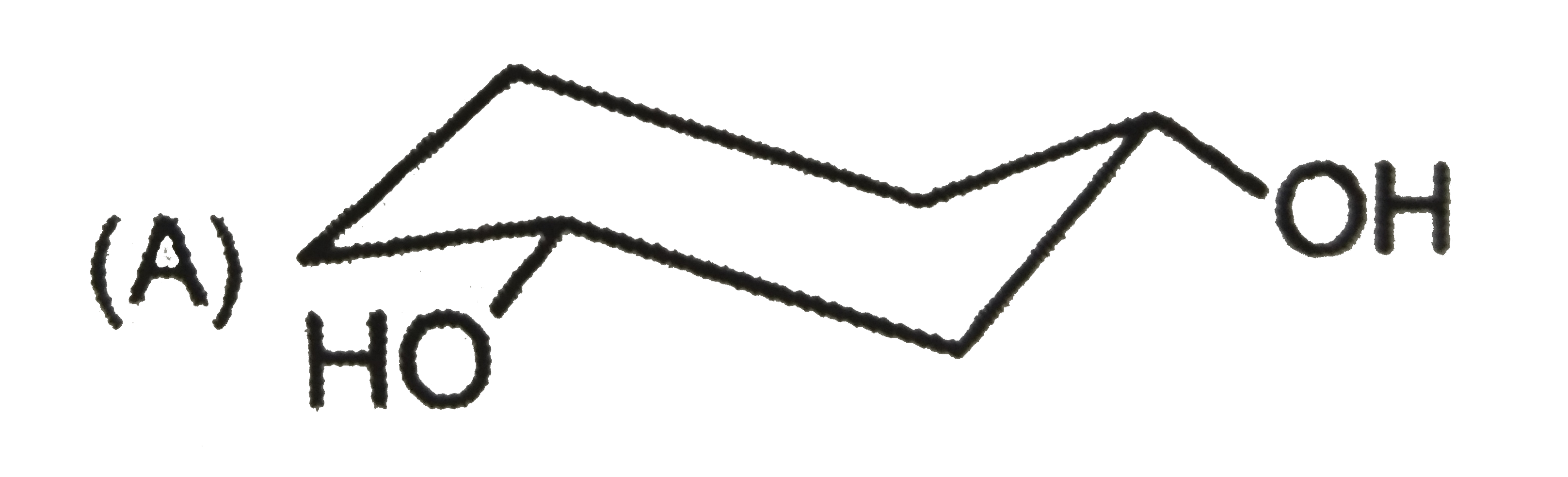
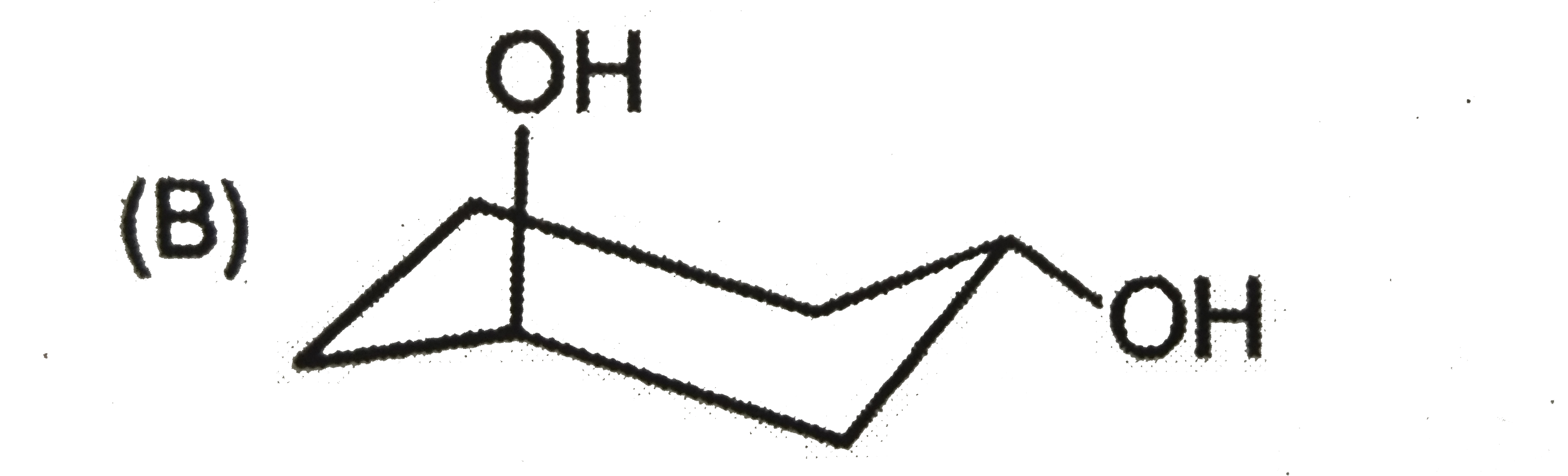
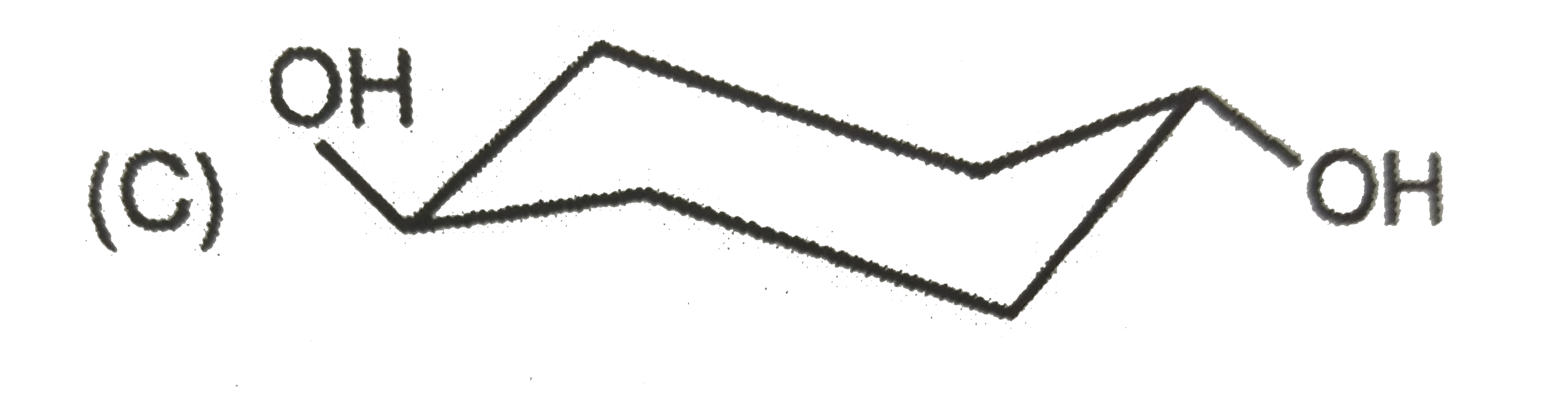
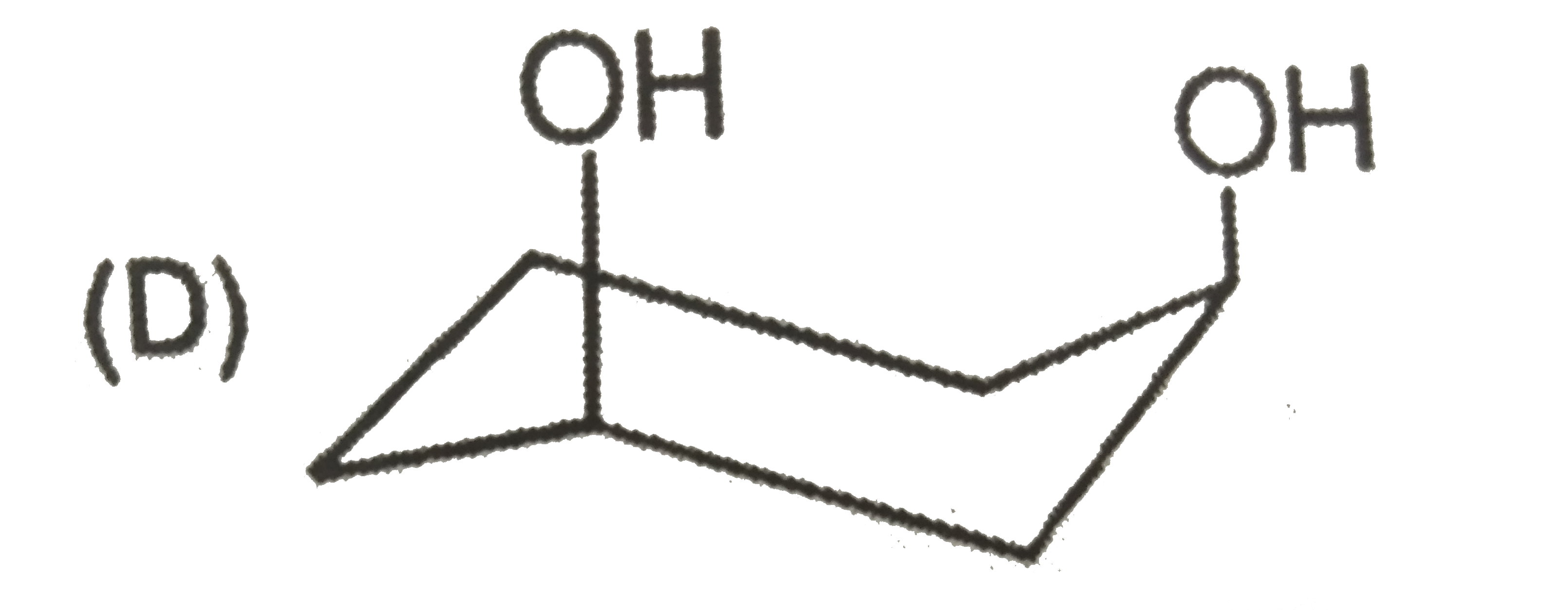
To determine the most stable form of cis cyclohexane-1,3-diol, we need to analyze the different conformations of the molecule. Here’s a step-by-step solution:
### Step 1: Understand the Structure
Cis cyclohexane-1,3-diol has two hydroxyl (-OH) groups located on the 1st and 3rd carbon atoms of the cyclohexane ring. The "cis" designation indicates that these groups are on the same side of the ring.
### Step 2: Identify Possible Conformations
In cyclohexane, substituents can occupy two types of positions: axial (pointing up or down from the plane of the ring) and equatorial (pointing outwards from the ring). For 1,3-diol, we can have:
- Axial - Axial (both -OH groups in axial positions)
- Equatorial - Equatorial (both -OH groups in equatorial positions)
- Axial - Equatorial (one -OH group in axial and one in equatorial)
### Step 3: Analyze Stability
1. **Axial - Axial Conformation**: When both -OH groups are in axial positions, they experience steric hindrance due to 1,3-diaxial interactions. However, they can also form hydrogen bonds with nearby hydrogen atoms, which can stabilize the structure.
2. **Equatorial - Equatorial Conformation**: In this conformation, both -OH groups are in equatorial positions, which minimizes steric hindrance. However, they are too far apart to form hydrogen bonds effectively, leading to a less stable configuration.
3. **Axial - Equatorial Conformation**: This configuration has one -OH group in an axial position and the other in an equatorial position. This arrangement can lead to some steric strain but allows for some hydrogen bonding.
### Step 4: Compare the Stability
- The **axial - axial** configuration, despite the steric strain, allows for hydrogen bonding, which can overcome the energy barriers created by the 1,3-diaxial interactions.
- The **equatorial - equatorial** configuration is less stable due to the lack of hydrogen bonding.
- The **axial - equatorial** configuration is also less stable than the axial - axial due to the presence of steric strain.
### Conclusion
The most stable form of cis cyclohexane-1,3-diol is the **axial - axial** conformation because the hydrogen bonding can effectively stabilize the structure despite the steric hindrance.
### Final Answer
The most stable form of cis cyclohexane-1,3-diol is represented as **D (axial - axial)**.
---