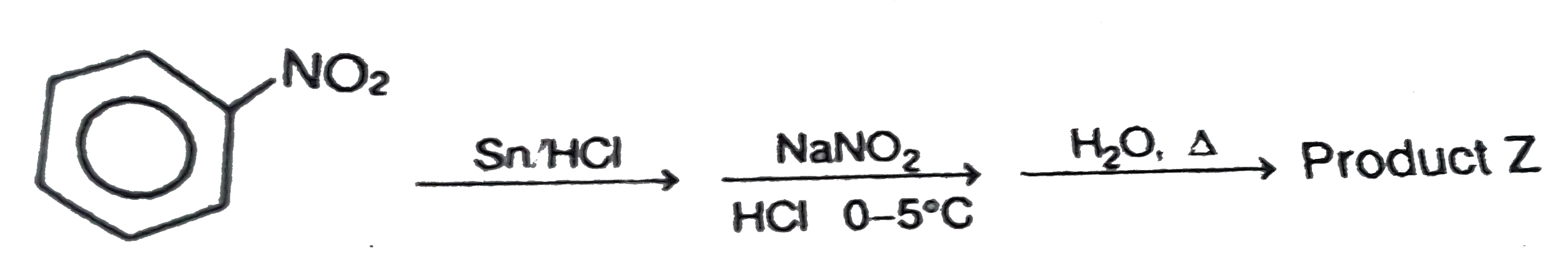Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
AROMATIC COMPOUNDS
RESONANCE ENGLISH|Exercise Part-III : One or more than one option correct type|10 VideosAROMATIC COMPOUNDS
RESONANCE ENGLISH|Exercise Part - IV Comprehension|13 VideosAROMATIC COMPOUNDS
RESONANCE ENGLISH|Exercise Exercise -2 (Part-I Only one option correct type)|12 VideosALKYL HALIDE, ALCOHOL, PHENOL, ETHER
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Alkyl Halide, Alcohol,Phenol,Ether)|56 VideosBASIC CONCEPTS
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(BASIC CONCEPTS)|27 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-AROMATIC COMPOUNDS-Part - II Single or double integer type
- Identify molecular weight of final product (Y).
Text Solution
|
- How many toluidines on reaction with NaNO(2)//HCl followed by H(3)PO(2...
Text Solution
|
- Find the molecular weight of Z.
Text Solution
|
- Molecular weight of T will be:
Text Solution
|
- How many N atom are present in final product.
Text Solution
|
- Ph-NO(2) overset(Sn//HCl)rarr underset("HCl, " 0^(@)C-5^(@)C)overset(N...
Text Solution
|
- In the given reaction how many of the following products (1-9) can be ...
Text Solution
|