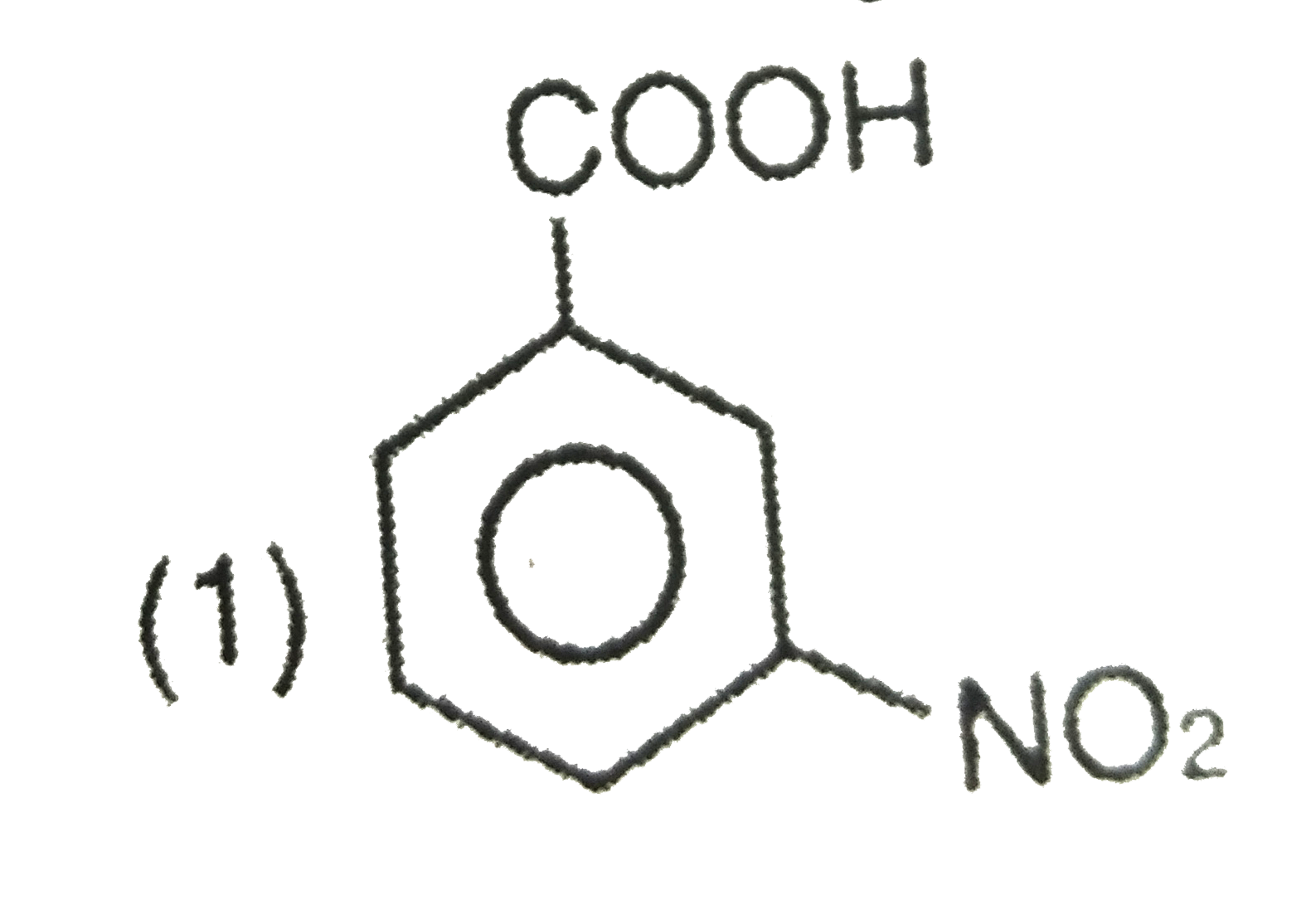
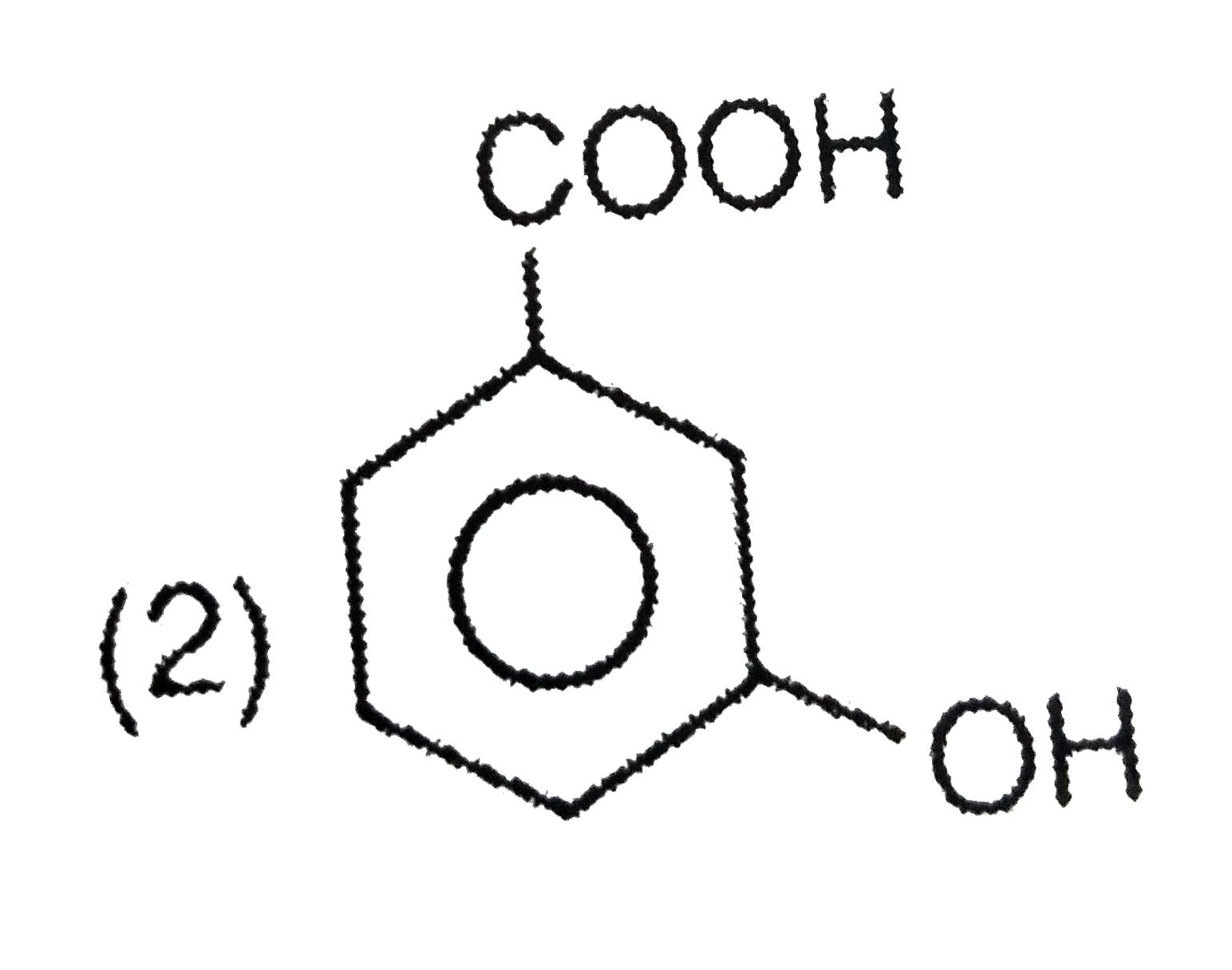
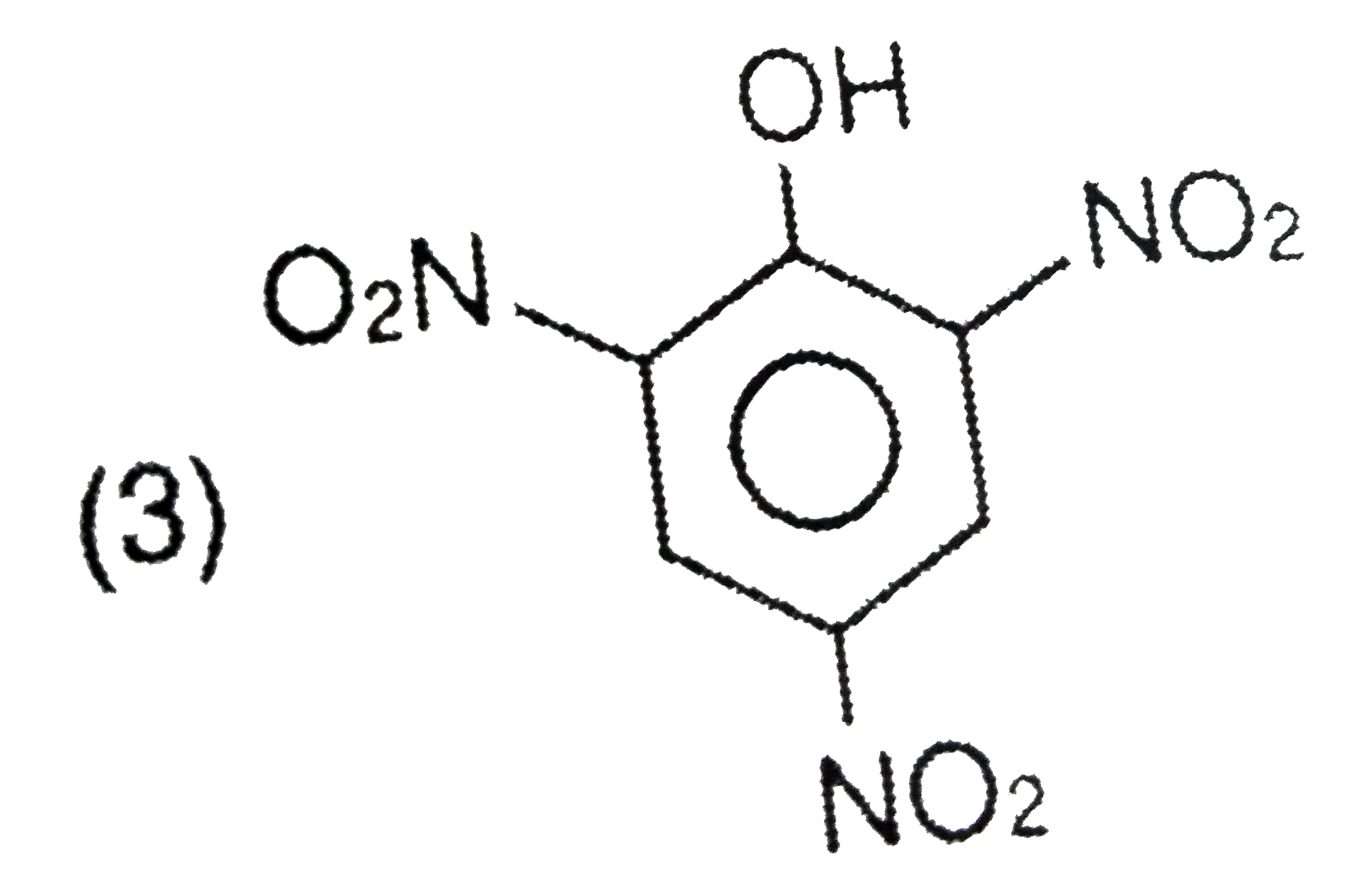
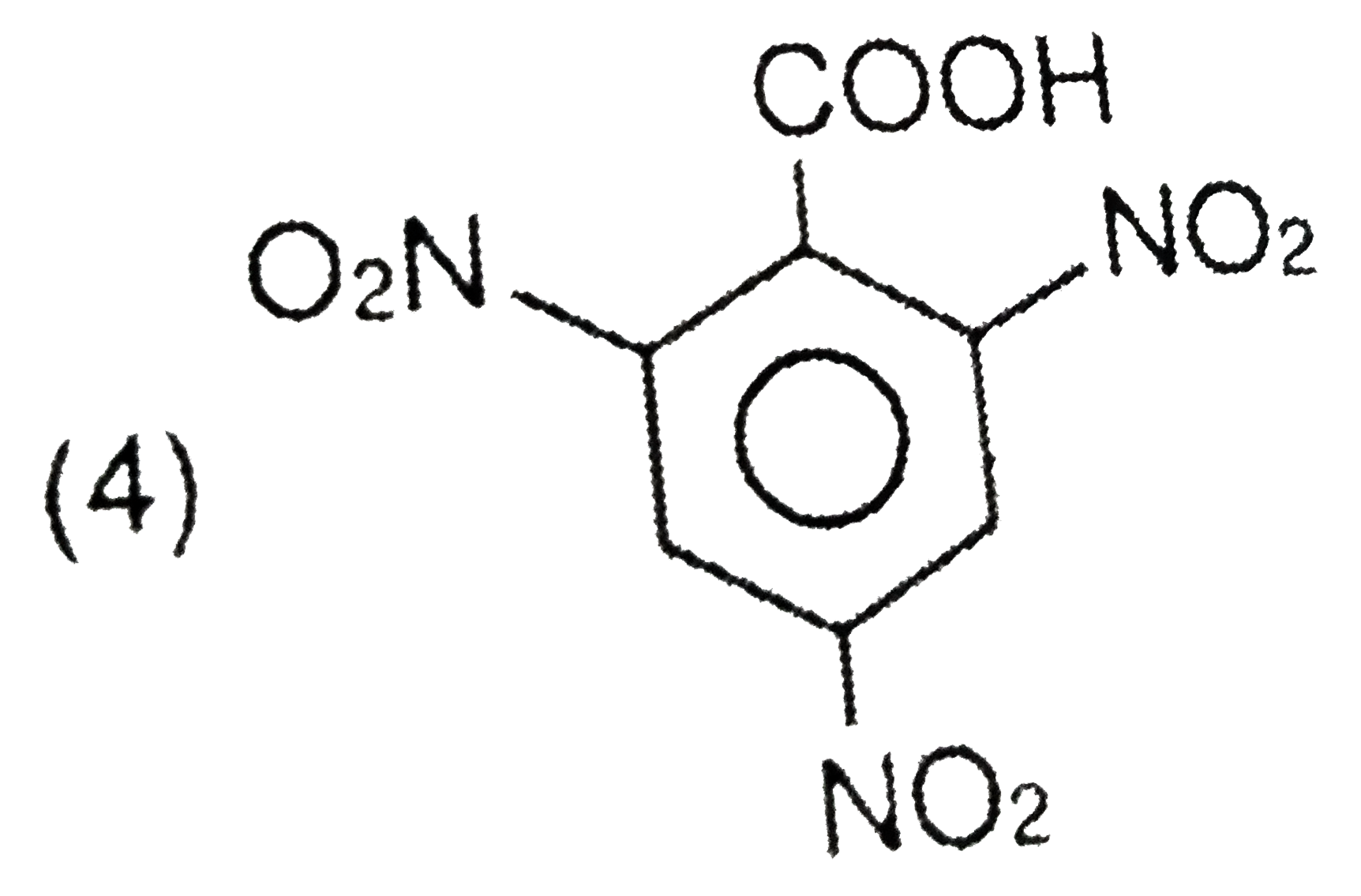
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
AROMATIC COMPOUNDS
RESONANCE ENGLISH|Exercise JEE MAIN (Online Problem)|15 VideosAROMATIC COMPOUNDS
RESONANCE ENGLISH|Exercise APSP (Part-I)|30 VideosAROMATIC COMPOUNDS
RESONANCE ENGLISH|Exercise Exercise-3 (Part-I) JEE Advanced|34 VideosALKYL HALIDE, ALCOHOL, PHENOL, ETHER
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Alkyl Halide, Alcohol,Phenol,Ether)|56 VideosBASIC CONCEPTS
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(BASIC CONCEPTS)|27 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-AROMATIC COMPOUNDS-Part - II JEE MAIN
- Picric acid is
Text Solution
|
- When primary amine reacts with chloroform in ethanolic KOH then produc...
Text Solution
|
- The reaction of chloroform with alcoholic KOH and p-toluidine form-
Text Solution
|
- Fluorobenzene (C(6)H(5)F) can be synthesized in the laboratory
Text Solution
|
- Which of the following compounds will give tribromo derivative on tre...
Text Solution
|
- The electrophile involved in the above reaction is:
Text Solution
|
- In the chemical reaction CH3CH2NH2+CHCl3+3KOHto(A), The +(B)+3H2O ...
Text Solution
|
- Phenol, when it first reacts with concentrated sulpuric acid and then ...
Text Solution
|
- Toluene is nitrated and the resulting product is reduced with tin and ...
Text Solution
|
- The major product obtained on interaction of phenol with sodium hydrox...
Text Solution
|
- In the chemical reactions the compounds (A) and (B) are .
Text Solution
|
- Phenol is heated with a solution of mixture of KBr and KBrO(3). The ma...
Text Solution
|
- In the following chemical reactions, the compounds A and B are respect...
Text Solution
|
- An organic compound A upon reacting with NH3 gives B. On heating B giv...
Text Solution
|
- Sodium phenoxide when heated with CO(2) under pressure at 125^@C yiel...
Text Solution
|
- In the reaction : the product E is
Text Solution
|
- In the Hofmann bromamide degradation reaction, the number of moles of ...
Text Solution
|
- Which of the following compounds will form significant amount of meta ...
Text Solution
|
- Phenol reacts with methyl chloroformate in the pressence of NaOH to f...
Text Solution
|
- Phenol on treatment with CO(2) in the presence of NaOH followed by aci...
Text Solution
|