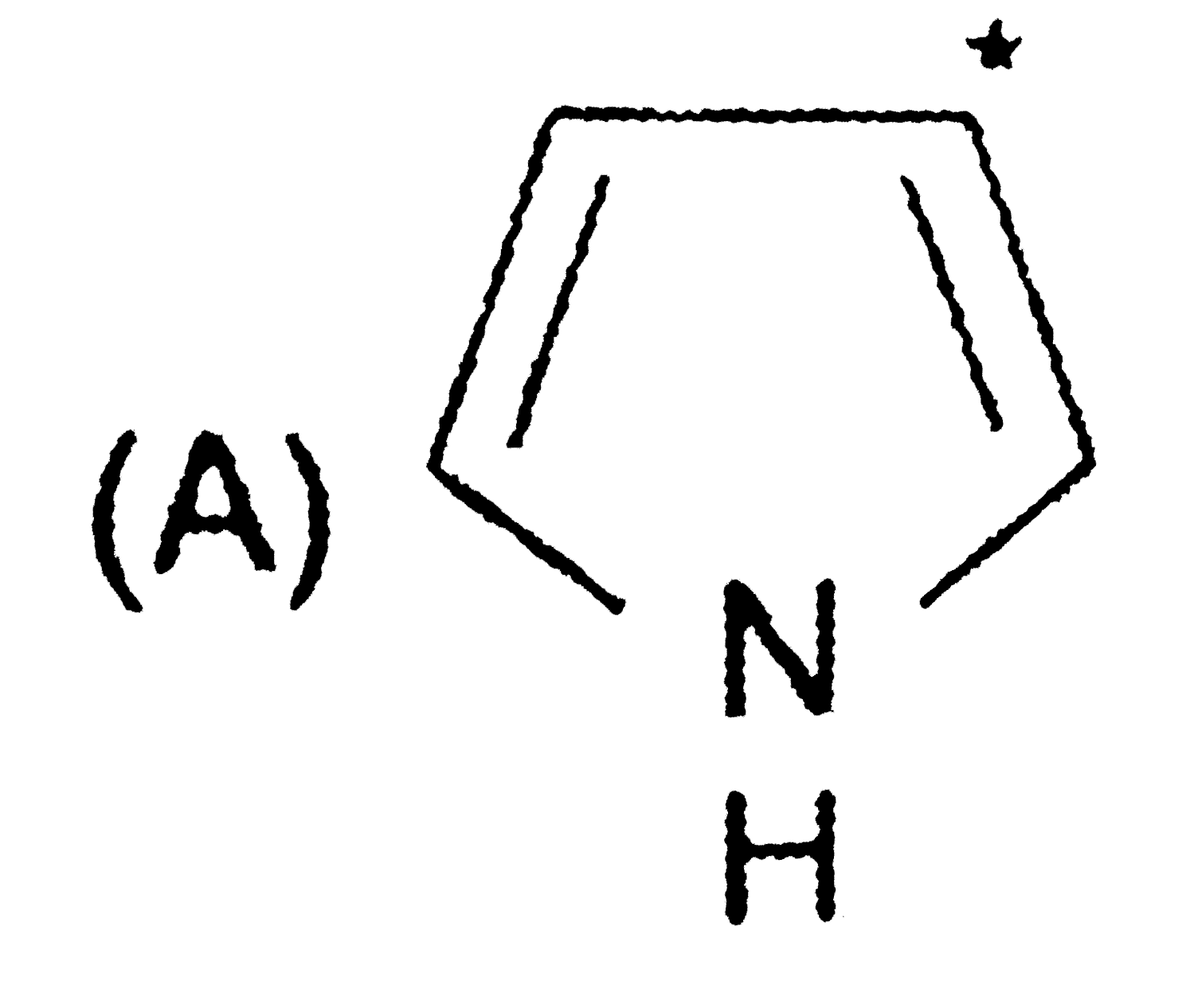
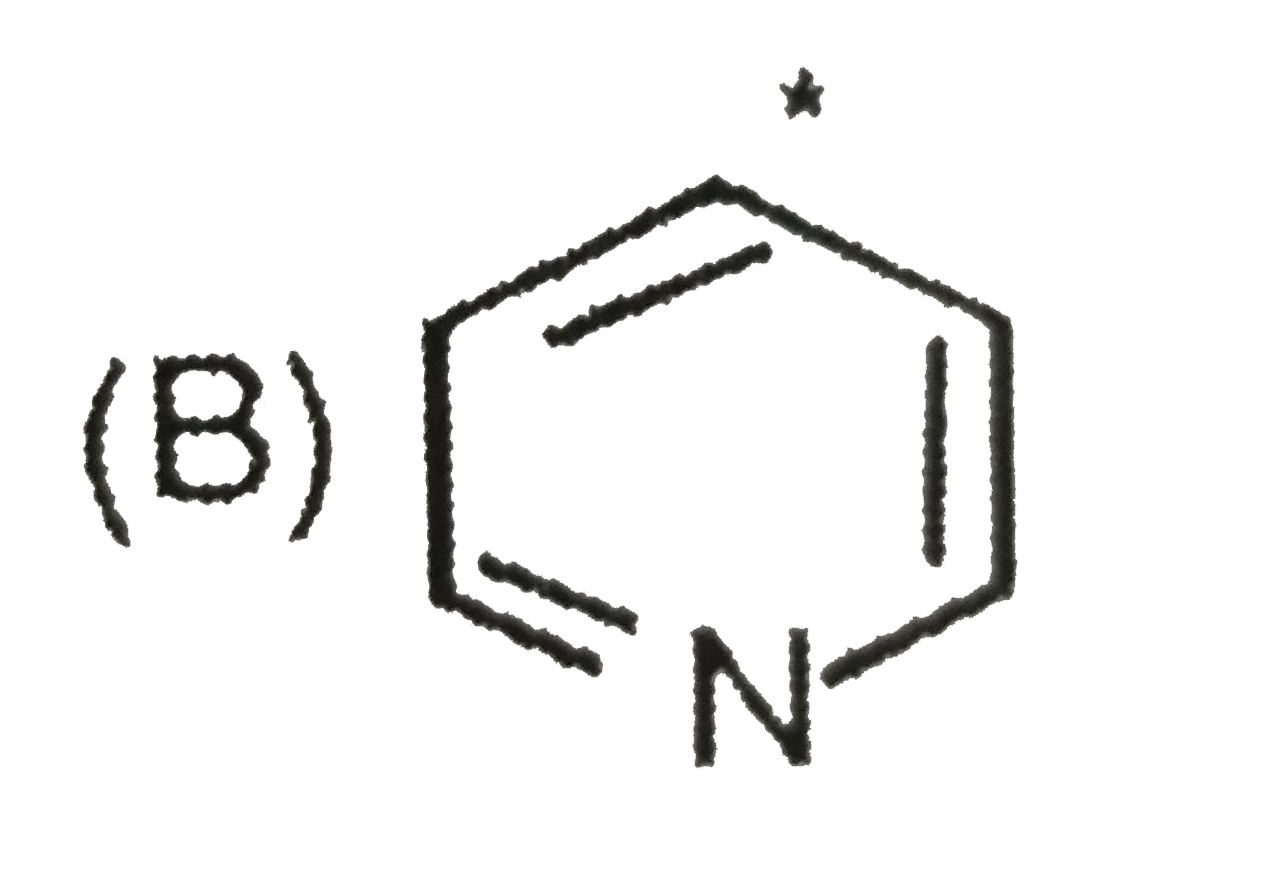
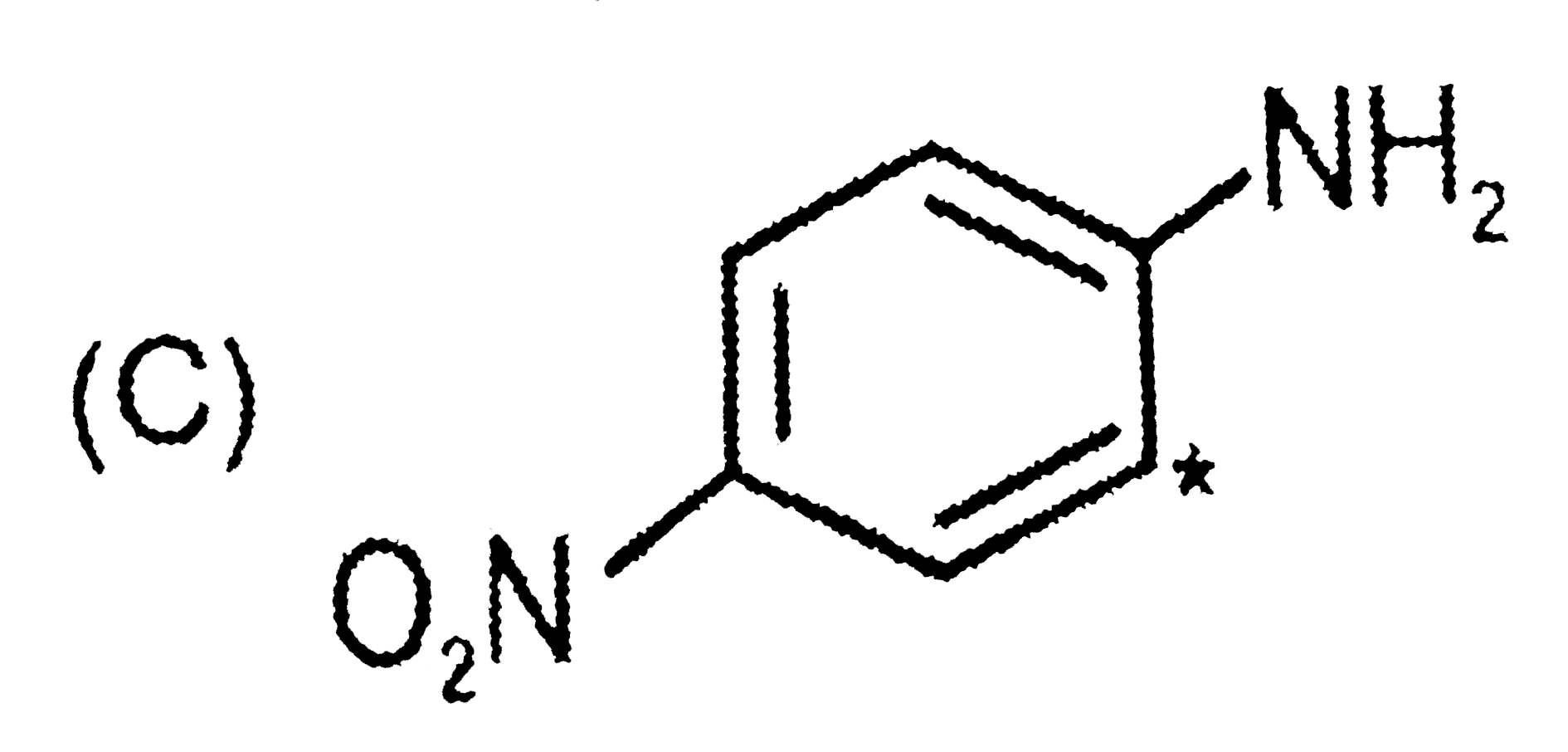
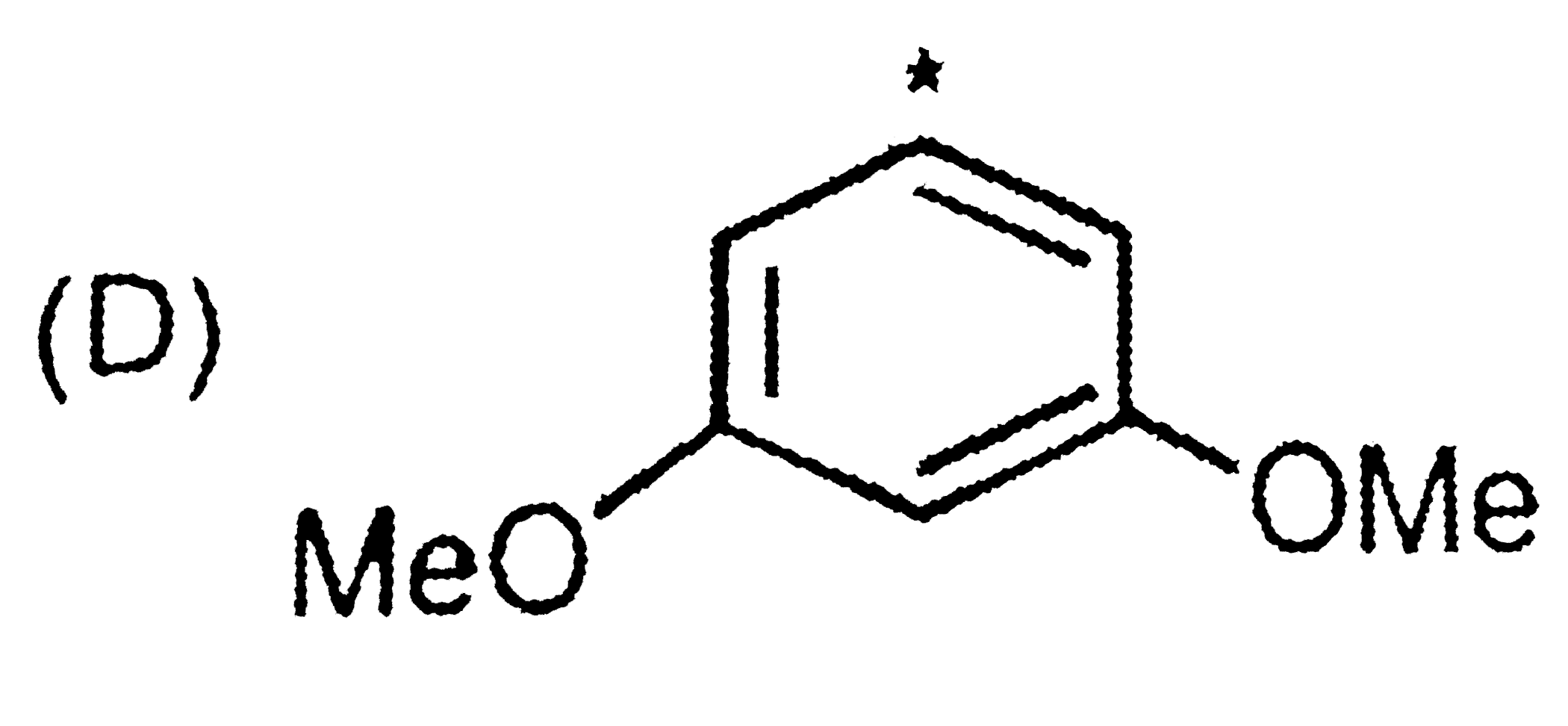
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
AROMATIC COMPOUNDS
RESONANCE ENGLISH|Exercise Part - III Practice Test-2|1 VideosAROMATIC COMPOUNDS
RESONANCE ENGLISH|Exercise Part - III Practice Test-3|1 VideosAROMATIC COMPOUNDS
RESONANCE ENGLISH|Exercise APSP (Part-I)|30 VideosALKYL HALIDE, ALCOHOL, PHENOL, ETHER
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Alkyl Halide, Alcohol,Phenol,Ether)|56 VideosBASIC CONCEPTS
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(BASIC CONCEPTS)|27 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-AROMATIC COMPOUNDS-Part - II NSEC
- p-chlorobenzoic acid can be prepared by reacting p-aminbenzoic acid wi...
Text Solution
|
- n-propylamine containing no secondary and tertiary amines as impuritie...
Text Solution
|
- The most favourable position (indicated by) for an electrophilic attac...
Text Solution
|
- The compound that on treatment with benzene sulphonyl chloride, forms ...
Text Solution
|
- The substance that gives a primary amine on hydrolysis is
Text Solution
|
- Toluene o/p orienting with respect to an electrophilic substitution re...
Text Solution
|
- The product obtained when 4-hydroxyhenzene sulphonic acid is treated w...
Text Solution
|
- The most appropriate reaction for the conversion of bromobenzene to be...
Text Solution
|
- P-bromoanine is prepared from aniline via
Text Solution
|
- Which one of the following has the highest melting point?
Text Solution
|
- On bromination, the electron rich phenoxide ion will be attacked most ...
Text Solution
|
- The sequence of decreasing aromaticity in the above compounds is
Text Solution
|
- Can the amino group, in the aniline molecule, become meta-directing in...
Text Solution
|
- Consider the following reactions the major product (Y) of the re...
Text Solution
|
- In mechanism of Reimer- Riemann reaction, species involves is
Text Solution
|
- The nitrogen atom in the following cyclic compounds can be removed as ...
Text Solution
|
- X overset(Mg)underset("ether")toY overset("Dry CO(2))underset(H^(+))to...
Text Solution
|
- Salicylic acid on treatment with bromine water will give
Text Solution
|
- The product P obtained through the following sequence of reactions is
Text Solution
|
- Triethylamine is reacted with a peracid to obtain X. The nitrogen atom...
Text Solution
|