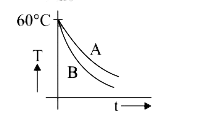
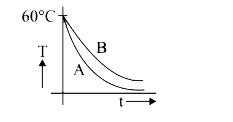
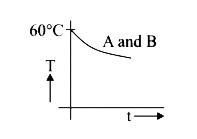
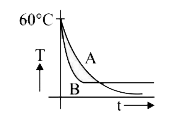
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-JEE MAIN REVISION TEST - 10| JEE -2020-PHYSICS (SECTION 2)
- Two identical beakers A and B contain equal volumes of two different l...
Text Solution
|
- For the circuit shown, with R(1)=1.0Omega,R(2)=2.0Omega,E(1)=2V and E(...
Text Solution
|
- In an interference experiment the ratio of amplitudes of coherent wave...
Text Solution
|
- A particle moves in one dimension from rest under the influence of a f...
Text Solution
|
- The reverse breakdown voltage of a Zener diode is 5.6V in the given ci...
Text Solution
|
- A 200Omega resistor has a certain color code. If one replaces the red ...
Text Solution
|