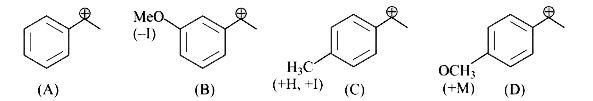
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-JEE MAIN REVISION TEST -14 -CHEMISTRY (SECTON 2)
- Rate of S(N)1 reactin for the following compounds is: (a). (b). ...
Text Solution
|
- The graph for an orbital between |varphi|^(2) and r(radial distance) i...
Text Solution
|
- The number of S-S bonds in H(2)S(4)O(6) is
Text Solution
|
- How many of the following are activated towards electrophilic aromatic...
Text Solution
|
- At room temperature, a dilute solution of urea is prepared by dissolvi...
Text Solution
|
- The number of bonds between carbon and oxygen atoms in major product o...
Text Solution
|
_2019_CBT5_E01_001_Q01.png)
_2019_CBT5_E01_001_Q02.png)
_2019_CBT5_E01_001_Q03.png)
_2019_CBT5_E01_001_Q04.png)