Text Solution
Verified by Experts
Topper's Solved these Questions
STOICHIOMETRY - I
VMC MODULES ENGLISH|Exercise Level - 0 (Short Answer Type -II)|6 VideosSTOICHIOMETRY - I
VMC MODULES ENGLISH|Exercise Level -0 (Long Answer Type)|7 VideosSTOICHIOMETRY - I
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|31 VideosSTATES OF MATTER
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE-I|10 VideosSTOICHIOMETRY-II
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|43 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-STOICHIOMETRY - I-(Level - 0 (Short Answer Type - I)
- How many significant figures should be present in the answer of the fo...
Text Solution
|
- A compound on analysis was found to contain C = 34.6 %, H = 3.85 % and...
Text Solution
|
- How many moles of methane are required to produce 22g of CO(2) on comb...
Text Solution
|
- Dinitrogen and dihydrogen react with each other to produce ammonia acc...
Text Solution
|
- What volumes of 10 M HCl and 3 M HCl should be mixed to get 1L of 6 M ...
Text Solution
|
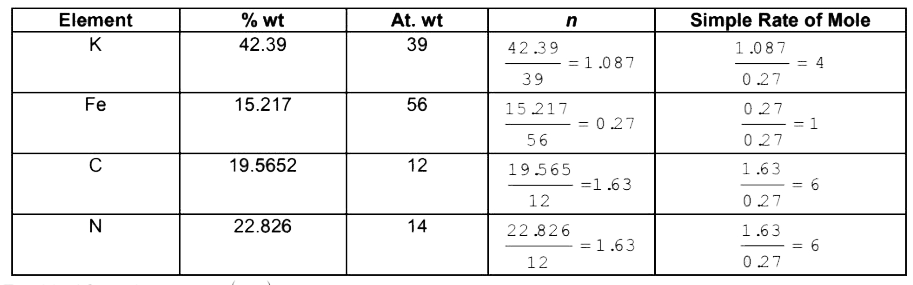
- A compound contains 42.3913 % K,15.2173 % Fe,19.5652 % C and 22.8260 %...
Text Solution
|
- Calculate the amount of carbon dioxide that could be produced when ...
Text Solution
|