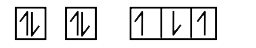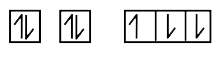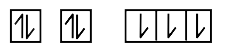A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC STRUCTURE
VMC MODULES ENGLISH|Exercise LEVEL - 2|50 VideosATOMIC STRUCTURE
VMC MODULES ENGLISH|Exercise LEVEL - 2 ( Numerical value type for JEE Main )|16 VideosATOMIC STRUCTURE
VMC MODULES ENGLISH|Exercise Level - 0 (Long Answer Type (5 Marks))|11 VideosAMINES
VMC MODULES ENGLISH|Exercise IN CHAPTER EXERCISE H|10 VideosCHEMICAL BONDING & CHEMICAL STRUCTURE
VMC MODULES ENGLISH|Exercise IMPECCABLE|50 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-ATOMIC STRUCTURE-LEVEL - 1
- The number of orbitals and subshells present in the shell with n = 4 i...
Text Solution
|
- The number of electrons in the valence shell of calcium is:
Text Solution
|
- The ground state electronic configuration of nitrogen atom can be repr...
Text Solution
|
- How many unpaired electrons are present in Ni^(2+) cation? (At. No. = ...
Text Solution
|
- An electron, a proton and an alpha particle have KE of 16E, 4E and E r...
Text Solution
|
- Which of the following sets of quantum numbers represents the highest ...
Text Solution
|
- The number of radial nodes of 3s and 2s orbital are respectively:
Text Solution
|
- In hydrogen atom an orbit has a diameter of about 16.92 Å, what is the...
Text Solution
|
- The number of waves in n^("th") orbit are:
Text Solution
|
- The magnitude of the spin angular momentum of an electron is given by:
Text Solution
|
- Which of the following sets of quantum number is INCORRECT? (I) n=3,...
Text Solution
|
- The correct set of four quantum numbers for outermost electron of pota...
Text Solution
|
- A body of mass x kg is moving with a velocity of 100ms^(-1) . Its de-B...
Text Solution
|
- The values of four quantum number of valence electron of an element ar...
Text Solution
|
- The orbital angular momentum of an electron in a d-orbital is:
Text Solution
|
- de-Broglie wavelength of electron in 2^("nd") excited state of hydroge...
Text Solution
|
- The H-spectrum show:
Text Solution
|
- Electrons will first enter into which set of quantum numbers-n = 5, l ...
Text Solution
|
- Which of the following configurations is incorrect?
Text Solution
|
- Which of the following set of quantum numbers is an impossible arrange...
Text Solution
|