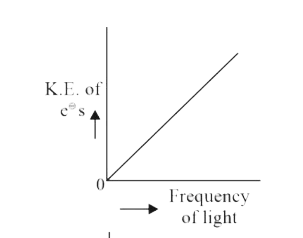
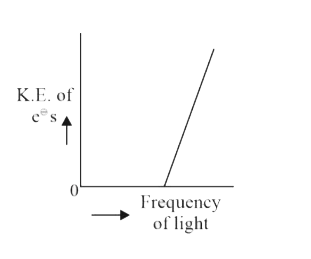
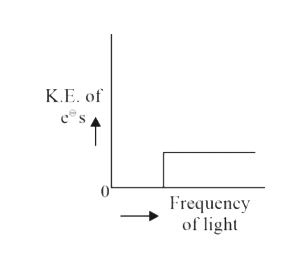
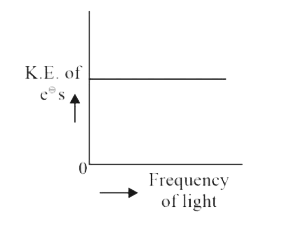
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ATOMIC STRUCTURE
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive )|67 VideosATOMIC STRUCTURE
VMC MODULES ENGLISH|Exercise LEVEL - 2 ( Numerical value type for JEE Main )|16 VideosAMINES
VMC MODULES ENGLISH|Exercise IN CHAPTER EXERCISE H|10 VideosCHEMICAL BONDING & CHEMICAL STRUCTURE
VMC MODULES ENGLISH|Exercise IMPECCABLE|50 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-ATOMIC STRUCTURE-JEE Main (Archive)
- The total number of orbitals associated with the principal quantum num...
Text Solution
|
- A stream of electrons from a heated filament was passed between two ch...
Text Solution
|
- The radius of the second Bohr orbit for hydrogen atom is [Planck's con...
Text Solution
|
- If the shortest wavelength in Lyman series of hydrogen atom is A, then...
Text Solution
|
- The electron in the hydrogen atom undergoes transition from higher orb...
Text Solution
|
- Ejection of the photoelectron from metal in the photoelectric effect e...
Text Solution
|
- The de Broglie's wavelength of electron present in first Bohr orbit of...
Text Solution
|
- Which of the following statements is false?
Text Solution
|
- For emission line of atomic hydrogen from n(i)=8 to n(f)=n, the plot o...
Text Solution
|
- Which of the following combination of statements is true regarding the...
Text Solution
|
- The ground state energy of hydrogen atom is -13.6 eV. The energy of se...
Text Solution
|
- Heat treatment of muscular pain involves radiation of wavelength of ab...
Text Solution
|
- what is the work fuction of the metal if the light of wavelength 4...
Text Solution
|
- If the de Broglie wavelength of the electron in n^(th) Bohr orbit in a...
Text Solution
|
- Which of the graphs shown below does not represent the relationship be...
Text Solution
|
- The de Broglie wavelenght (lambda) associated with a photoelectron var...
Text Solution
|
- The electrons are more likely to be found:
Text Solution
|
- In which of the following, energy of 2s orbital is minimum
Text Solution
|
- Which one of the following about an electron occupying the 1s orbital ...
Text Solution
|
- If p is the momentum of the fastest electron ejected from a metal surf...
Text Solution
|