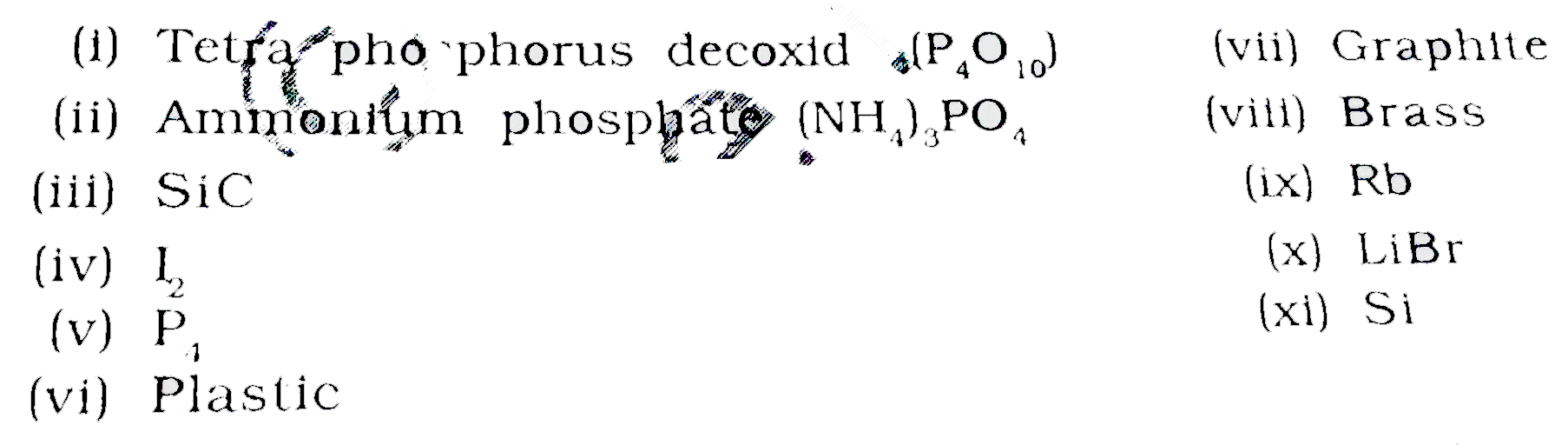Text Solution
Verified by Experts
Topper's Solved these Questions
THE SOLID STATE
VMC MODULES ENGLISH|Exercise LEVEL-0 ( Long Answer Type)|6 VideosTHE SOLID STATE
VMC MODULES ENGLISH|Exercise LEVEL-1|75 VideosTHE SOLID STATE
VMC MODULES ENGLISH|Exercise EXERCISE-J|10 VideosSURFACE CHEMISTRY
VMC MODULES ENGLISH|Exercise PRACTICE EXERCISE|9 VideosTHEORY OF SOLUTIONS
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|31 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-THE SOLID STATE-LEVEL-0 ( Short Answer Type)
- An element with molar mass 2.7 xx 10^-2 kg mol^(-1) forms a cubic unit...
Text Solution
|
- What type of defect can arise when a solid is heated? Which physical p...
Text Solution
|
- Explain how vacancies are introduced in an ionic solid when a cation ...
Text Solution
|
- Ionic solids. Which have anionic vacancies due to metal excess defect,...
Text Solution
|
- What type of substances would make better permanenet magnets, ferromag...
Text Solution
|
- What makes a glass different from a solid such as quartz? Under what c...
Text Solution
|
- classify each of the following solids as ionic, metallic, molecular, n...
Text Solution
|
- a. "Stability of a crystal is reflected in the magnitude of its melti...
Text Solution
|
- A cubic solid is made of two element P and Q Atoms of Q are the corner...
Text Solution
|
- Niobium crystallises in body-centred cubic structure if density is 8.5...
Text Solution
|
- Analysis shows that nickel oxide has the formula Ni(0.00). What fracti...
Text Solution
|
- Copper crystallizer into an fcc lattice with edge length 3.61 xx 10^(-...
Text Solution
|
- Non-stoichiometric cuprous oxide, Cu(2)O can be prepared in lboratory....
Text Solution
|
- Ferric oxide crystallises in a hexagonal close-packed array of oxide i...
Text Solution
|