A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
THE SOLID STATE
VMC MODULES ENGLISH|Exercise LEVEL-2|50 VideosTHE SOLID STATE
VMC MODULES ENGLISH|Exercise LEVEL-2 (Numerical Value Type for JEE Main )|15 VideosTHE SOLID STATE
VMC MODULES ENGLISH|Exercise LEVEL-0 ( Long Answer Type)|6 VideosSURFACE CHEMISTRY
VMC MODULES ENGLISH|Exercise PRACTICE EXERCISE|9 VideosTHEORY OF SOLUTIONS
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|31 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-THE SOLID STATE-LEVEL-1
- The ability of a substance to exist in two or more crystalline forms k...
Text Solution
|
- Which of the following statements about amorphous solid is incorrect ?
Text Solution
|
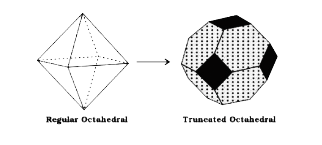
- Find the number of hexagonal faces that are present in a truncated oct...
Text Solution
|
- The number of atoms contained in a fcc unit cell of a monoatomic subst...
Text Solution
|
- The unit cell with parameter alpha=beta=gamma=90^(@) and a=b ne c is
Text Solution
|
- Assertion (A) : Graphite is an example of tetragonal crystal system. ...
Text Solution
|
- Na and Mg crystallize in bcc- and fcc-type crystals, respectively, the...
Text Solution
|
- In crystalline solids, which of the following element of symmetry is n...
Text Solution
|
- Aluminium metal has face centred cubic (f.c.c) structure. The number o...
Text Solution
|
- The axial angles in triclinic crystal system are
Text Solution
|
- How many kinds of space lattices are possible in a crystal?
Text Solution
|
- Ice crystallises in hexagonal lattice having the volume of unit cell a...
Text Solution
|
- Edge length of M^(+)X^(-) (fcc structure) is 7.2^(@)A. Assuming M^(+)-...
Text Solution
|
- The arrangement ABC ABC….is referred to as
Text Solution
|
- The coordination number of Al in the crystalline state of AlCl(3) is .
Text Solution
|
- Which of the following statement are correct for the ionic solids in w...
Text Solution
|
- The lattice points of a crystal of hydrogen iodide are occupied by :
Text Solution
|
- Point out the correct statement for the set of characteristics of ZnS ...
Text Solution
|
- A compound of A and B crystallizes in a cubic lattice in which A atoms...
Text Solution
|
- Arrangement of sulphide ions in zinc blende is :
Text Solution
|