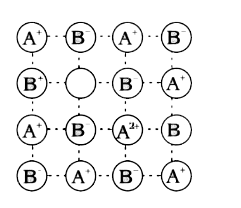
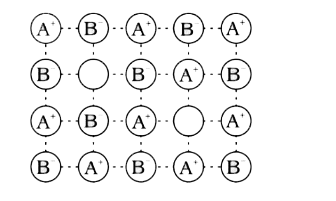
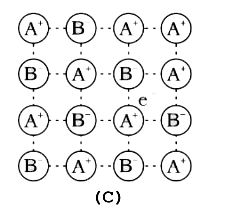
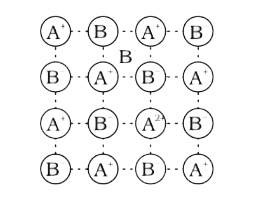
A
B
C
D
Text Solution
AI Generated Solution
Topper's Solved these Questions
THE SOLID STATE
VMC MODULES ENGLISH|Exercise LEVEL-2|50 VideosTHE SOLID STATE
VMC MODULES ENGLISH|Exercise LEVEL-2 (Numerical Value Type for JEE Main )|15 VideosTHE SOLID STATE
VMC MODULES ENGLISH|Exercise LEVEL-0 ( Long Answer Type)|6 VideosSURFACE CHEMISTRY
VMC MODULES ENGLISH|Exercise PRACTICE EXERCISE|9 VideosTHEORY OF SOLUTIONS
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|31 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-THE SOLID STATE-LEVEL-1
- How many types of Bravais lattices can occur in crystalline solids?
Text Solution
|
- The efficiency of packing in simple cubic unit cell is
Text Solution
|
- Which of the following represents incorrect representation of defect i...
Text Solution
|
- Lithium borohydride crystallizes in an orthorhombic system with 4 mole...
Text Solution
|
- KCl crystallises in the same type of lattice as does NaCl Given that r...
Text Solution
|
- The number of atoms in a cubic based unit cell having one atom on each...
Text Solution
|
- The density of a metal which crystallises in bcc lattice with unit cel...
Text Solution
|
- The unit cell of aluminium is a cube with an edge length of 405 pm. Th...
Text Solution
|
- The radius ratio (r(+)//r(-)) of an ionic solid (A^(+)B^(-)) is 0.69. ...
Text Solution
|
- A metal has bcc structure and the edge length of its unit cell is 3.04...
Text Solution
|
- In orthorhombic , the value of a, b and c are respectively 4.2Å, 8.6 Å...
Text Solution
|
- Identify the incorrect statement regarding a crystal showing Schottky ...
Text Solution
|
- Three elements P,Q and R crystallize in a cubic solid lattice. The P a...
Text Solution
|
- If a cation leaves a site in solid lattice, and is located at an inter...
Text Solution
|
- Schottky defect occurs mainly in electrovalent compounds where
Text Solution
|
- Which of the following statements is correct?
Text Solution
|
- In a face centred cubic arrangement of A and B atoms whose A atoms are...
Text Solution
|
- Which of the following defects, if present, lowers the density of the ...
Text Solution
|
- Schottky defect is generally observed in :
Text Solution
|
- The flame colours of metal ions are due to
Text Solution
|