A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
THE SOLID STATE
VMC MODULES ENGLISH|Exercise JEE Advance ( Archive )|27 VideosTHE SOLID STATE
VMC MODULES ENGLISH|Exercise FUNDAMENTAL|50 VideosTHE SOLID STATE
VMC MODULES ENGLISH|Exercise LEVEL-2 (Numerical Value Type for JEE Main )|15 VideosSURFACE CHEMISTRY
VMC MODULES ENGLISH|Exercise PRACTICE EXERCISE|9 VideosTHEORY OF SOLUTIONS
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|31 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-THE SOLID STATE-JEE Main (Archive )
- A metal crystallises in a face centred cubic structure. If the edge le...
Text Solution
|
- A ionic compound has a unit cell consisting of a ions at the corners o...
Text Solution
|
- CsCl crystallises in body centred cubic lattice. If 'a' is its edge le...
Text Solution
|
- Sodium metal crystallizes in a body centred cubic lattice with a unit ...
Text Solution
|
- Which of the following compounds is metallic and ferromagnetic ?
Text Solution
|
- A metal crystallises in a face centred cubic structure. If the edge le...
Text Solution
|
- Which type of defect has the presence of cations in the interstitial s...
Text Solution
|
- The one that is extensively used as a piezoelectric material is
Text Solution
|
- At 100^(@), copper (Cu) has FCC unit cell structure with cell edge len...
Text Solution
|
- A compound of formula A(2)B(3) has the hcp lattice. Which atom forms t...
Text Solution
|
- A solid having density of 9 xx 10^(3) kg m^(-3) forms face centred cub...
Text Solution
|
- Which premitive unit cell has unequual edge lengths (anebnec) and all ...
Text Solution
|
- The radius of the largest shpere which fits properly at the centre of ...
Text Solution
|
- A forms ccp lattice B occupy half of the octahedral voids and O occupy...
Text Solution
|
- An element crystallises in a face -centred cubic (fcc) unit cell with...
Text Solution
|
- The ratio of number of atoms present in a simple cubic, body centered ...
Text Solution
|
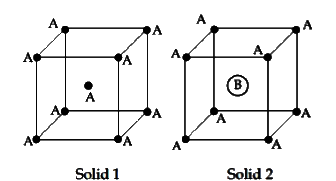
- Consider the bcc unit cells of the solids 1 and 2 with the position of...
Text Solution
|
- Which of the following is incorrect about interstitial compounds.
Text Solution
|
- Which of the following compounds is likely to show both Frenkel a...
Text Solution
|
- A substance 'X' having low melting point, does not conduct electricity...
Text Solution
|