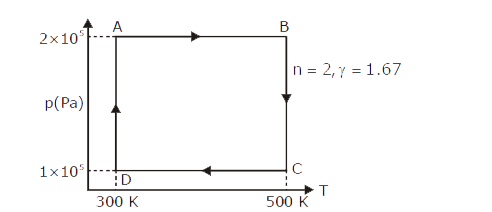
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GASEOUS STATE & THERMODYNAMICS
VMC MODULES ENGLISH|Exercise JEE ADVANCED (ARCHIVE )|111 VideosGASEOUS STATE & THERMODYNAMICS
VMC MODULES ENGLISH|Exercise Level - 2|40 VideosENERGY & MOMENTUM
VMC MODULES ENGLISH|Exercise JEE ADVANCE (ARCHIVE) - TRUE/FALSE TYPE|1 VideosGRAVITATION
VMC MODULES ENGLISH|Exercise JEE Advance (Archive) TRUE/FALSE|1 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-GASEOUS STATE & THERMODYNAMICS-JEE MAIN (ARCHIVE )
- An insulated container of gas has two chambers separated by an insulat...
Text Solution
|
- One kg of a diatomic gas is at a pressure of 8 xx 10^(4) N//m^(2). The...
Text Solution
|
- Assume the gas to be ideal, the work done on the gas in taking it from...
Text Solution
|
- Two moles of helium gas are taken over the cycle ABCDA , as shown in t...
Text Solution
|
- Two moles of helium gas are taken over the cycle ABCDA , as shown in t...
Text Solution
|
- A diatomic ideal gas is used in a Carnot engine as the working substan...
Text Solution
|
- 100g of water is heated from 30^@C to 50^@C. Ignoring the slight expan...
Text Solution
|
- Three perfect gases at absolute temperature T(1), T(2) and T(3) are mi...
Text Solution
|
- A thermally insulated vessel contains an ideal gas of molecular mass M...
Text Solution
|
- A Carnot engine operating between temperature T1 and T2 has efficiency...
Text Solution
|
- The specific heat capacity of a metal at low temperature (T) is given ...
Text Solution
|
- A container with insulating walls is divided into two equal parts by a...
Text Solution
|
- Helium gas goes through a cycle ABCDA (consisting of two isochoric and...
Text Solution
|
- A Carnot engine, whose efficiency is 40%, takes in heat from a source ...
Text Solution
|
- The above p-v diagram represents the thermodynamic cycle of an engine,...
Text Solution
|
- One mole of diatomic ideal gas undergoes a cyclic process ABC as shown...
Text Solution
|
- An open glass tube is immersed in mercury in such a way that a length ...
Text Solution
|
- Consider an ideal gas confined in an isolated closed chamber. As the g...
Text Solution
|
- Consider a spherical shell of radius R at temperature T. The black bod...
Text Solution
|
- Which of the following is incorrect regarding the first law of thermod...
Text Solution
|