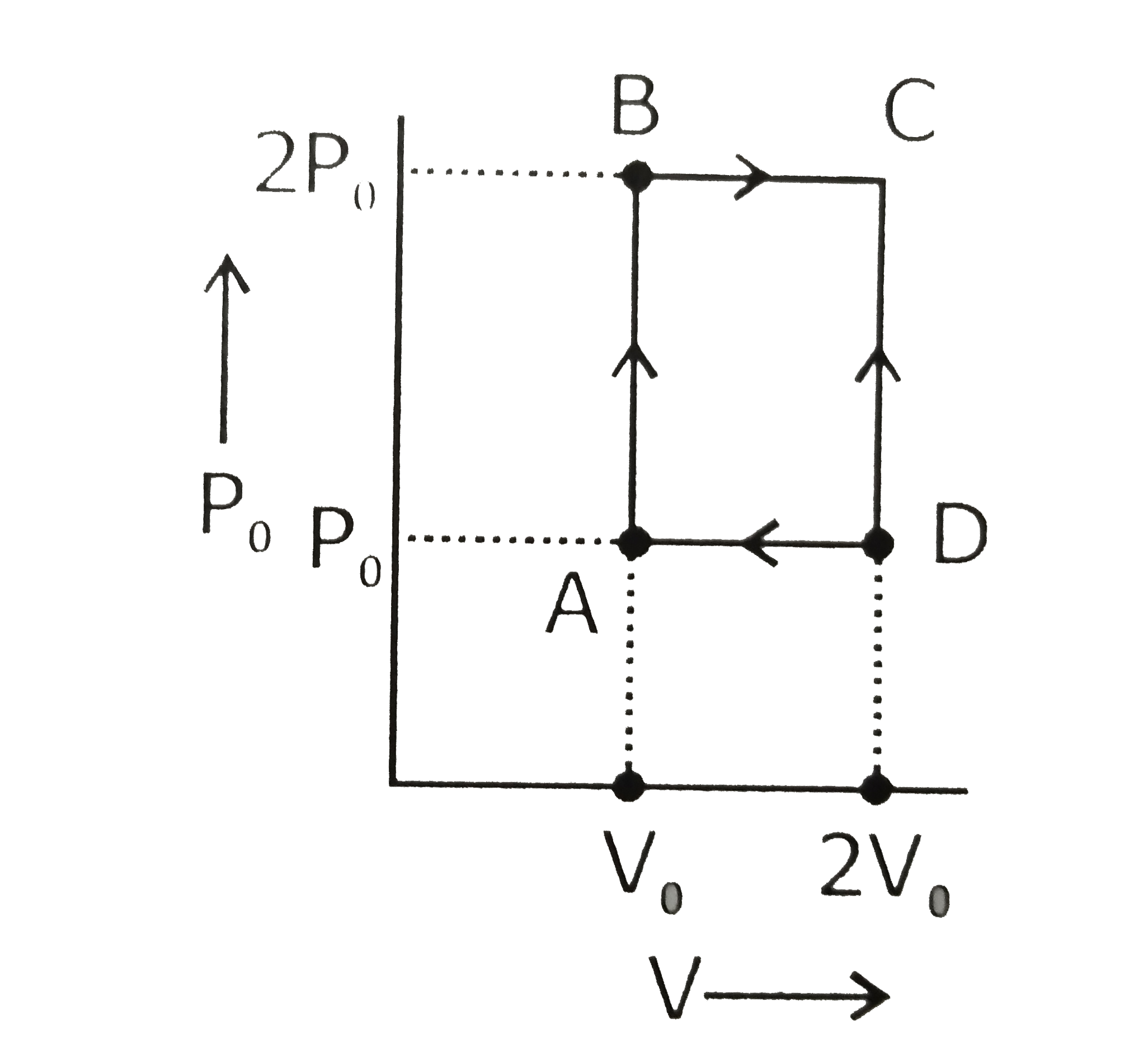
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GASEOUS STATE & THERMODYNAMICS
VMC MODULES ENGLISH|Exercise JEE ADVANCED (ARCHIVE )|111 VideosGASEOUS STATE & THERMODYNAMICS
VMC MODULES ENGLISH|Exercise Level - 2|40 VideosENERGY & MOMENTUM
VMC MODULES ENGLISH|Exercise JEE ADVANCE (ARCHIVE) - TRUE/FALSE TYPE|1 VideosGRAVITATION
VMC MODULES ENGLISH|Exercise JEE Advance (Archive) TRUE/FALSE|1 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-GASEOUS STATE & THERMODYNAMICS-JEE MAIN (ARCHIVE )
- An ideal gas has molecules with 5 degrees of freedom. The ratio of spe...
Text Solution
|
- N moles of an ideal diatomic gas are in a cylinder at temperature T. s...
Text Solution
|
- An engine operates by taking n moles of an ideal gas through the cycle...
Text Solution
|
- For the diagram given for an ideal gas, out of the following which o...
Text Solution
|
- The temperature of an open room of volume 30 m^(3) increases from 17^(...
Text Solution
|
- One mole of and ideal monoatomic gas is compressed isothermally in a...
Text Solution
|
- The value closest to the thermal velocity of a helium atom at room tem...
Text Solution
|
- Two moles of helium are mixed with n moles of hydrogen. If (Cp)/(Cv)=(...
Text Solution
|
- A Carnot's engine works as refrigerator between 250 K and 300K. It r...
Text Solution
|
- The mass of hydrogen molecule is 3.32xx10^(-27) kg. If 10^(23) hydroge...
Text Solution
|
- Two moles of an ideal monatomic gas occupies a volume V at 27 °C. The ...
Text Solution
|
- Two Camot engines A and B are operated in series. The first one, A , r...
Text Solution
|
- A mass M = 15 g of nitrogen is enclosed in a vessel at temperature T =...
Text Solution
|
- A mixture of 2 moles of helium gas ( (atomic mass)=4a.m.u ) and 1 mole...
Text Solution
|
- A gas can be ken from A to B via two different processes ACB and ADB. ...
Text Solution
|
- Two kg of a monoatomic gas is at a pressure of 4 xx 10^(4) N//m^(2). T...
Text Solution
|
- Half mole of an ideal monoatomic gas is heated at constant pressure of...
Text Solution
|
- Three Carnot engines operate in series between a heat source at a temp...
Text Solution
|
- In a process, temperature and volume of one mole of an ideal monoato...
Text Solution
|
- A gas mixture consists of 3 moles of oxygen and 5 moles of argon at te...
Text Solution
|