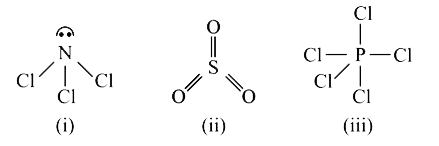
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING-I & II
VMC MODULES ENGLISH|Exercise Level - 2 (JEE Advanced)|50 VideosCHEMICAL BONDING-I & II
VMC MODULES ENGLISH|Exercise JEE Main (Numerical Value Questions)|15 VideosCHEMICAL BONDING-I & II
VMC MODULES ENGLISH|Exercise Level - 0 (Long Answer Type)|4 VideosCHEMICAL BONDING & MOLECULAR STRUCTURE
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE-L|9 VideosCHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE - G|10 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-CHEMICAL BONDING-I & II-Level - 1 (JEE Main)
- Which set contain no ionic species?
Text Solution
|
- Which of the following has zero dipole moment?
Text Solution
|
- Which are non-polar molecules? I. NCl(3) " "II. SO(3)" ...
Text Solution
|
- Which of the following ions has the maximum polarising power?
Text Solution
|
- All the following molecules are polar except one
Text Solution
|
- Which of the following has the highest percentage of ionic character i...
Text Solution
|
- Which of the following is non-polar but contains polar bonds?
Text Solution
|
- The N(2)H(4) molecule contains :
Text Solution
|
- Which statement is not correct about NO(2) ?
Text Solution
|
- The hybrid orbital of the central atom in AlF(4)^(-) is :
Text Solution
|
- Select the correct statement.
Text Solution
|
- In which of the following set do all the three compunds have bonds tha...
Text Solution
|
- Which of the following bonds is most polar ?
Text Solution
|
- The C-H bond distance is the longest in:
Text Solution
|
- Which of the following molecules does not have net dipole moment?
Text Solution
|
- Correct order of bond length is
Text Solution
|
- The hybridization of the central atom in ClO(2)F(3) is :
Text Solution
|
- Which of the following molecules has the maximum value of bond energy ...
Text Solution
|
- Which have linear structure?
Text Solution
|
- What is the geometry of BrF(3)?
Text Solution
|