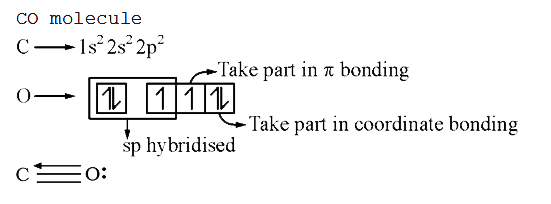A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING-I & II
VMC MODULES ENGLISH|Exercise Level - 2 (JEE Advanced)|50 VideosCHEMICAL BONDING-I & II
VMC MODULES ENGLISH|Exercise JEE Main (Numerical Value Questions)|15 VideosCHEMICAL BONDING-I & II
VMC MODULES ENGLISH|Exercise Level - 0 (Long Answer Type)|4 VideosCHEMICAL BONDING & MOLECULAR STRUCTURE
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE-L|9 VideosCHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE - G|10 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-CHEMICAL BONDING-I & II-Level - 1 (JEE Main)
- Which of the following molecules or ions is not linear?
Text Solution
|
- In carbon-hydrogen-oxygen compounds :
Text Solution
|
- The shapes of XeO(2)F(2) molecule is
Text Solution
|
- Which of the following ions has resonating structures?
Text Solution
|
- Covalency of carbon in the CO molecule is three because
Text Solution
|
- Which has maximum number of lone pair of electrons present on central ...
Text Solution
|
- The nitrogen atom in NH(3), NH(2)^(-) and NH(4)^(+) are all surrounde...
Text Solution
|
- Which of the following has the highest boiling point?
Text Solution
|
- The correct order of dipole moments of HF, H(2)S and H(2)O is :
Text Solution
|
- The correct order of increasing bond length of C-H, C-O, C - C and C =...
Text Solution
|
- Which of the following compound or ion is planar?
Text Solution
|
- Explain why PCl(5) is trigonal bipyramidal whereas IF(5) is square pyr...
Text Solution
|
- In diborane (B(2)H(6)), the bond formed between B and B is called :
Text Solution
|
- Which of the following pairs have identical value of bond order?
Text Solution
|
- In Be(2) the bond order is
Text Solution
|
- The number of anti bonding electrons in N(2) is
Text Solution
|
- A simplified applified of MO theory to the hypotheritical molecule OF ...
Text Solution
|
- Which of the following pairs have identical value of bond order?
Text Solution
|
- Paramagnetism is exhibited by :
Text Solution
|
- N(2) and O(2) are converted into mono anions, N(2)^(-) and O(2)^(-) ...
Text Solution
|