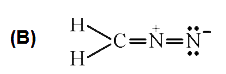
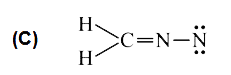
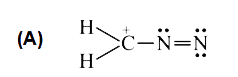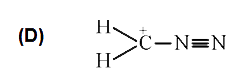A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING-I & II
VMC MODULES ENGLISH|Exercise JEE Main (Numerical Value Questions)|15 VideosCHEMICAL BONDING-I & II
VMC MODULES ENGLISH|Exercise JEE Main (Archive)|62 VideosCHEMICAL BONDING-I & II
VMC MODULES ENGLISH|Exercise Level - 1 (JEE Main)|75 VideosCHEMICAL BONDING & MOLECULAR STRUCTURE
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE-L|9 VideosCHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE - G|10 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-CHEMICAL BONDING-I & II-Level - 2 (JEE Advanced)
- In the linear I3^(-) (triiodide ion), the central iodine atom contains
Text Solution
|
- In the sp^(3)d hybridisation of the central atom having two lone pairs...
Text Solution
|
- Which of the two do you think is more important contributor to the res...
Text Solution
|
- Select the incorrect statement.
Text Solution
|
- Which of these molecules have non-bonding electron pairs on the centra...
Text Solution
|
- Which species has the same shape as the NO(3)^(-) ion?
Text Solution
|
- The hybridisation scheme for the central atom includes a d-orbital con...
Text Solution
|
- AsF(5) molecule is trigonal bipyramidal. The orbitals of As atom invol...
Text Solution
|
- In a P(4) molecule, the P – P – P bond angle is :
Text Solution
|
- Hybridisation of Boron in B(2)H(6) molecule is :
Text Solution
|
- Which of the following molecule have same structure and shape ?
Text Solution
|
- Match List-1 and List-II and pick out correct matching codes from of g...
Text Solution
|
- Which carbon is more electronegative ?
Text Solution
|
- How many hydrogen bonds can be formed by a water molecule?
Text Solution
|
- In which reaction, the hybridisation on the central atom changes from ...
Text Solution
|
- In which of the following there exists a p pi-p pi bonding
Text Solution
|
- Which of the following are true ?
Text Solution
|
- In which of the following change in hybridisation is taking place ?
Text Solution
|
- The structure and hybridisation of Si(CH(3))(4) is
Text Solution
|
- In which of the following there is maximum p pi -p pi bonding ?
Text Solution
|