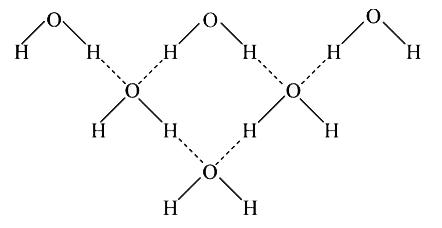
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING-I & II
VMC MODULES ENGLISH|Exercise JEE Main (Numerical Value Questions)|15 VideosCHEMICAL BONDING-I & II
VMC MODULES ENGLISH|Exercise JEE Main (Archive)|62 VideosCHEMICAL BONDING-I & II
VMC MODULES ENGLISH|Exercise Level - 1 (JEE Main)|75 VideosCHEMICAL BONDING & MOLECULAR STRUCTURE
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE-L|9 VideosCHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE - G|10 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-CHEMICAL BONDING-I & II-Level - 2 (JEE Advanced)
- Hybridisation of Boron in B(2)H(6) molecule is :
Text Solution
|
- Which of the following molecule have same structure and shape ?
Text Solution
|
- Match List-1 and List-II and pick out correct matching codes from of g...
Text Solution
|
- Which carbon is more electronegative ?
Text Solution
|
- How many hydrogen bonds can be formed by a water molecule?
Text Solution
|
- In which reaction, the hybridisation on the central atom changes from ...
Text Solution
|
- In which of the following there exists a p pi-p pi bonding
Text Solution
|
- Which of the following are true ?
Text Solution
|
- In which of the following change in hybridisation is taking place ?
Text Solution
|
- The structure and hybridisation of Si(CH(3))(4) is
Text Solution
|
- In which of the following there is maximum p pi -p pi bonding ?
Text Solution
|
- The d-orbital involved in the hybridization of central atom in XeOF(2)...
Text Solution
|
- In which of the following ionisation processes, the bond order has inc...
Text Solution
|
- Which one of the following statements is correct ?
Text Solution
|
- In the conversion of N(2) into N(2)^(+) the electron will be lost fro...
Text Solution
|
- Which is the correct statement ?
Text Solution
|
- Which of the following have fractional bond order and is(are) paramagn...
Text Solution
|
- The sequence that correctly describes the relative bond strengths pert...
Text Solution
|
- The correct order of bond order values among the following (i) NO^(...
Text Solution
|
- Which of the following will participate in intermolecular H-bonding?
Text Solution
|