A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING-I & II
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|98 VideosCHEMICAL BONDING-I & II
VMC MODULES ENGLISH|Exercise JEE Main (Numerical Value Questions)|15 VideosCHEMICAL BONDING & MOLECULAR STRUCTURE
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE-L|9 VideosCHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE - G|10 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-CHEMICAL BONDING-I & II-JEE Main (Archive)
- The states of hybridisation of boron and oxygen atoms in boric acid (H...
Text Solution
|
- Which of the following has the regular tetrahedral structure?
Text Solution
|
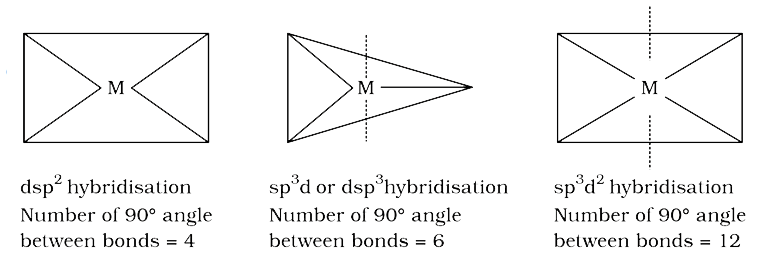
- The maximum number of 90^(@) angles between bond pair-bond pair of ele...
Text Solution
|
- Lattice energy of an ionic compound depends on
Text Solution
|
- Which of the following molecules/ins does not contain unpaired electro...
Text Solution
|
- In which of the following molecules /ions , are all the bonds not equa...
Text Solution
|
- The decreasing valuse of bond angles from NH(3) (106^(@)) to SbH(3)(10...
Text Solution
|
- Which of the following species exhibits the diamagnetic behaviour ?
Text Solution
|
- The charge/size ratio of a cation determines its polarizing power. Whi...
Text Solution
|
- In which of the following ionisation processes, the bond order has inc...
Text Solution
|
- Which of the following hydrogen bonds is the strongest ?
Text Solution
|
- Which one of the following pairs of species have the same bond order?
Text Solution
|
- The bond dissociation energy of B-F in BF(3) is 646 kJ mol^(-1) wherea...
Text Solution
|
- Using MO theory predict which of the following species has the shortes...
Text Solution
|
- Among the following, the maximum covalent character is shown by the co...
Text Solution
|
- The types of hybrid orbitals of nitrogen in NO(2)^(+), NO(3)^(-) and N...
Text Solution
|
- The structure of IF(7) is
Text Solution
|
- ortho- Nitrophenol is less soluble in water than p- and m- nitrophenol...
Text Solution
|
- In which of the following pairs the two species are not isotructural ?
Text Solution
|
- Stability of the species Li(2), Li(2)^(-) and Li(2)^(+) increases in t...
Text Solution
|