A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING-I & II
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|98 VideosCHEMICAL BONDING-I & II
VMC MODULES ENGLISH|Exercise JEE Main (Numerical Value Questions)|15 VideosCHEMICAL BONDING & MOLECULAR STRUCTURE
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE-L|9 VideosCHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE - G|10 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-CHEMICAL BONDING-I & II-JEE Main (Archive)
- The types of hybrid orbitals of nitrogen in NO(2)^(+), NO(3)^(-) and N...
Text Solution
|
- The structure of IF(7) is
Text Solution
|
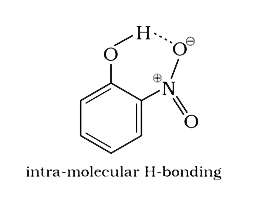
- ortho- Nitrophenol is less soluble in water than p- and m- nitrophenol...
Text Solution
|
- In which of the following pairs the two species are not isotructural ?
Text Solution
|
- Stability of the species Li(2), Li(2)^(-) and Li(2)^(+) increases in t...
Text Solution
|
- In which of the following pairs of molecules/ions, both the species ar...
Text Solution
|
- The correct statement for the molecule, Csl(3) is
Text Solution
|
- The intermolecular interaction that is dependent on the inverse cube o...
Text Solution
|
- The shape of "XeOF"(2) on the basis of VSEPR theory is
Text Solution
|
- Molecule AB has a bond length of 1.617Å and a dipole moment of 0.38 D....
Text Solution
|
- The species in which the N atom is in a state of sp hybridization is :
Text Solution
|
- After understanding the assertion and reason, choose the correct optio...
Text Solution
|
- Choose the incorrect formula out of the four compounds for an element ...
Text Solution
|
- The group of molecules having identical shape is:
Text Solution
|
- The bond angle H-X-H is the greatest in teh compound :
Text Solution
|
- Which of the following species is not paramagnetic?
Text Solution
|
- The group having isoelectronic species is
Text Solution
|
- Which of the following is paramagnetic ?
Text Solution
|
- The group having triangular planar structures is :
Text Solution
|
- Total number of lone pair of electrons in I(3)^(-) ion is
Text Solution
|