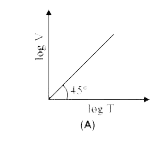
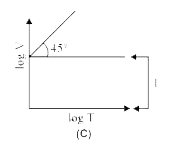
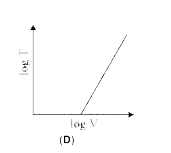
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
VMC MODULES ENGLISH|Exercise LEVEL-2|50 VideosTHERMODYNAMICS
VMC MODULES ENGLISH|Exercise LEVEL-2 (NUMERICAL VALUE TYPE)|15 VideosTHERMODYNAMICS
VMC MODULES ENGLISH|Exercise LEVEL-0 (LONG ANSWER TYPE (5 MARKS))|6 VideosTHERMOCHEMISTRY
VMC MODULES ENGLISH|Exercise JEE ADVANCED (ARCHIVE)|31 VideosTHERMODYNAMICS & THERMOCHEMISTRY
VMC MODULES ENGLISH|Exercise Impeccable|48 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-THERMODYNAMICS-LEVEL-1
- Consider the reaction at 300 K C(6) H(6) (l)+(15)/(2)O(2)(g)rarr6C...
Text Solution
|
- An ideal gas expands against a constant external pressure of 2.0 atmos...
Text Solution
|
- For a closed container containing 100 mole of an ideal gas fitted with...
Text Solution
|
- 10 mole of ideal gas expand isothermally and reversibly from a pressur...
Text Solution
|
- Under which of the following condition is the relation DeltaH = DeltaU...
Text Solution
|
- The work done in ergs for the reversible expansion of one mole of an i...
Text Solution
|
- The molar heat capacities at constant pressure (assumed constant with ...
Text Solution
|
- Consider the reaction at 300 K C(6) H(6) (l)+(15)/(2)O(2)(g)rarr6C...
Text Solution
|
- Give the name of the following reaction: C(2)H(5)Br+C(2)H(5)ON a to ...
Text Solution
|
- One mole of solid Zn is placed in excess of dilute H(2)SO(4) at 27^(@)...
Text Solution
|
- The enthalpy change (DeltaH) for the reaction N2(g) + 3H2 (g) to 2N...
Text Solution
|
- When 1 g of ice at 0^(@)C melts to form 1 g of water at 0^(@)C then, i...
Text Solution
|
- 130 g of Zn is dissolved in dilute sulphuric acid in an open beaker . ...
Text Solution
|
- The ammount of heat required to raise the temperature of 1 mole of dia...
Text Solution
|
- Calculat average molar heat capacity at constant volume of gaseous mix...
Text Solution
|
- In the isothermal reversible compression of 52.0m mol of a perfect gas...
Text Solution
|
- A sample of oxygen gas expands its volume from 3L to 5L against a cons...
Text Solution
|
- A : Work done by a gas in isothermal expension is more than the work d...
Text Solution
|
- Determine DeltaU^(@) at 300 K for the following reaction using the lis...
Text Solution
|
- From the given table answer the following questions: Reaction: H(...
Text Solution
|