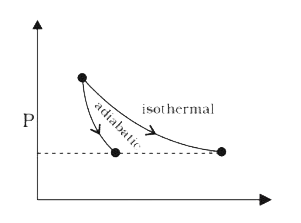
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
VMC MODULES ENGLISH|Exercise LEVEL-2|50 VideosTHERMODYNAMICS
VMC MODULES ENGLISH|Exercise LEVEL-2 (NUMERICAL VALUE TYPE)|15 VideosTHERMODYNAMICS
VMC MODULES ENGLISH|Exercise LEVEL-0 (LONG ANSWER TYPE (5 MARKS))|6 VideosTHERMOCHEMISTRY
VMC MODULES ENGLISH|Exercise JEE ADVANCED (ARCHIVE)|31 VideosTHERMODYNAMICS & THERMOCHEMISTRY
VMC MODULES ENGLISH|Exercise Impeccable|48 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-THERMODYNAMICS-LEVEL-1
- In the isothermal reversible compression of 52.0m mol of a perfect gas...
Text Solution
|
- A sample of oxygen gas expands its volume from 3L to 5L against a cons...
Text Solution
|
- A : Work done by a gas in isothermal expension is more than the work d...
Text Solution
|
- Determine DeltaU^(@) at 300 K for the following reaction using the lis...
Text Solution
|
- From the given table answer the following questions: Reaction: H(...
Text Solution
|
- Calculate the free energy change at 298 K for the reaction, Br(2)(l)...
Text Solution
|
- One mole of an ideal gas is expanded from a volume of 3L to 5L under a...
Text Solution
|
- For the reaction 2HgO(s) rarr 2Hg(l) + O(2)(g)
Text Solution
|
- Predict which of the following reaction(s) has a positive entropy chan...
Text Solution
|
- Which of the following is/are state function?
Text Solution
|
- The enthalpy of vaporisation of a liquid is 30 kJ mol^(-1) and entropy...
Text Solution
|
- When the gas is ideal and process is isothermal, then
Text Solution
|
- A system absorbs 300cal of heat , its volume doubles and temperature r...
Text Solution
|
- Temperature of one mole of a gas is increased by 1^(@) C at constant p...
Text Solution
|
- P-V plot for two gases (assuming ideal) during adiabatic processes are...
Text Solution
|
- Calculate the final temperature of a monoatomic ideal gas that is comp...
Text Solution
|
- The adsorption of vapours on a clean surface is a spontaneous process ...
Text Solution
|
- Column-I and Column-II contains four entries each. Entries of Column-I...
Text Solution
|
- Match Column-I with Column-II and select the correct answer using the ...
Text Solution
|
- A process is taking place at constant temperature and pressure. Then
Text Solution
|