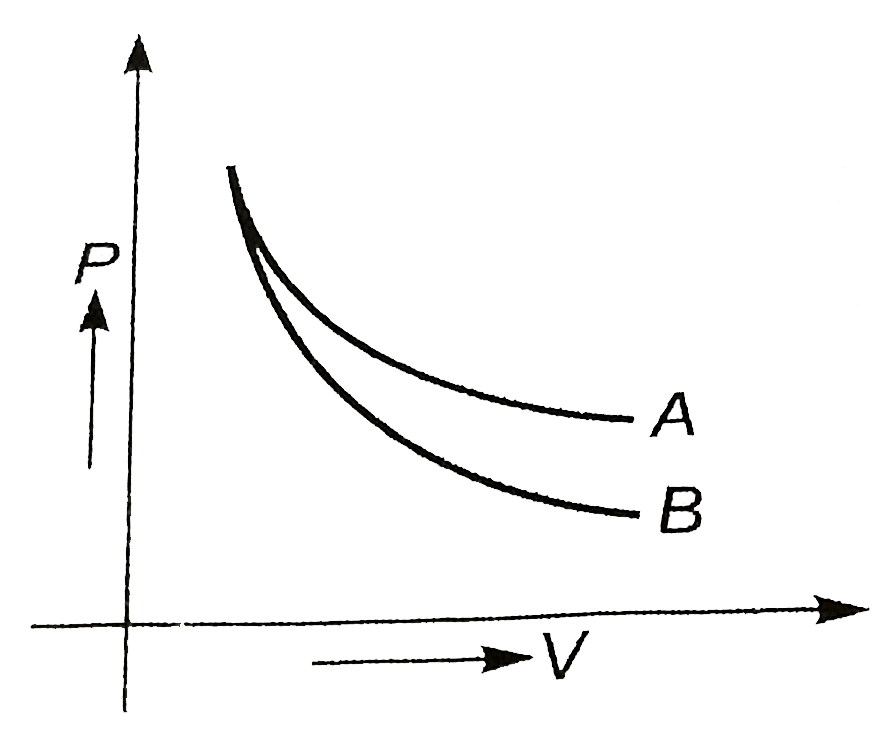
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
VMC MODULES ENGLISH|Exercise LEVEL-2|50 VideosTHERMODYNAMICS
VMC MODULES ENGLISH|Exercise LEVEL-2 (NUMERICAL VALUE TYPE)|15 VideosTHERMODYNAMICS
VMC MODULES ENGLISH|Exercise LEVEL-0 (LONG ANSWER TYPE (5 MARKS))|6 VideosTHERMOCHEMISTRY
VMC MODULES ENGLISH|Exercise JEE ADVANCED (ARCHIVE)|31 VideosTHERMODYNAMICS & THERMOCHEMISTRY
VMC MODULES ENGLISH|Exercise Impeccable|48 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-THERMODYNAMICS-LEVEL-1
- A system absorbs 300cal of heat , its volume doubles and temperature r...
Text Solution
|
- Temperature of one mole of a gas is increased by 1^(@) C at constant p...
Text Solution
|
- P-V plot for two gases (assuming ideal) during adiabatic processes are...
Text Solution
|
- Calculate the final temperature of a monoatomic ideal gas that is comp...
Text Solution
|
- The adsorption of vapours on a clean surface is a spontaneous process ...
Text Solution
|
- Column-I and Column-II contains four entries each. Entries of Column-I...
Text Solution
|
- Match Column-I with Column-II and select the correct answer using the ...
Text Solution
|
- A process is taking place at constant temperature and pressure. Then
Text Solution
|
- In view of the signs of DeltarG^@ for the following reactions PbO2 +...
Text Solution
|
- A plot of ln k against (1)/(T) (abscissa) is expected to be a straight...
Text Solution
|
- The correct relationship between free energy change in a reaction and ...
Text Solution
|
- For the reaction at 298 K A(g) + B(g) hArr C(g) + D(g) If DeltaH^(...
Text Solution
|
- एंटोपि का मात्रक है ---
Text Solution
|
- For a system in equilibrium, DeltaG=0, under conditions of constant
Text Solution
|
- Calculate the entropy change for the following reaction H(2)(g) +CI(...
Text Solution
|
- The free energy for a reaction having DeltaH=31400 cal, DeltaS=32" cal...
Text Solution
|
- Spontaneous adsorption of a gas on solid surface is an exothermic proc...
Text Solution
|
- The enthalpy change for transition of liquid water to steam is 40.8 kJ...
Text Solution
|
- Which of the following statements is true?
Text Solution
|
- In a reversible process, the value of Delta S (sys) + Delta S(surr) is
Text Solution
|