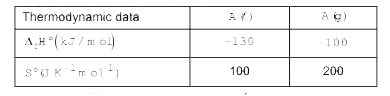A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
VMC MODULES ENGLISH|Exercise LEVEL-2|50 VideosTHERMODYNAMICS
VMC MODULES ENGLISH|Exercise LEVEL-2 (NUMERICAL VALUE TYPE)|15 VideosTHERMODYNAMICS
VMC MODULES ENGLISH|Exercise LEVEL-0 (LONG ANSWER TYPE (5 MARKS))|6 VideosTHERMOCHEMISTRY
VMC MODULES ENGLISH|Exercise JEE ADVANCED (ARCHIVE)|31 VideosTHERMODYNAMICS & THERMOCHEMISTRY
VMC MODULES ENGLISH|Exercise Impeccable|48 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-THERMODYNAMICS-LEVEL-1
- The enthalpy change for transition of liquid water to steam is 40.8 kJ...
Text Solution
|
- Which of the following statements is true?
Text Solution
|
- In a reversible process, the value of Delta S (sys) + Delta S(surr) is
Text Solution
|
- Consider the following cyclic process. I. Isothermal , II. Adiaba...
Text Solution
|
- The following diagram represents the (p-V) changes of gas. Thus, total...
Text Solution
|
- Which of the following proces is (are) expected to be spontaneous at h...
Text Solution
|
- Which of the plots of ln K vs (1/T) is/are correct?
Text Solution
|
- The value of DeltaH("transition") of C (graphite) rarr C (diamond) is ...
Text Solution
|
- Among the following , the state funcation (s) is (are)
Text Solution
|
- For an endothermic reaction, Delta H represents the enthalpy of the re...
Text Solution
|
- Match the following:
Text Solution
|
- Match the Column:
Text Solution
|
- For the gas phase reaction, PCl(5)(g) hArr PCl(3)(g) + Cl(2)(g) Wh...
Text Solution
|
- The Haber's process of production of ammonia involves the equilibrium:...
Text Solution
|
- If a gas, at constant temperature and pressure expands, then its
Text Solution
|
- Considering the reaction, C(s) + O(2)(g) to CO(2)(g) + 393.5 kJ th...
Text Solution
|
- Considering entropy (S) as a thermodynamic parameter, the criterion fo...
Text Solution
|
- Assuming DeltaH^(@) and S^(@) do not change with temperature. Calculat...
Text Solution
|
- For a phase change: H(2)O(l)hArrH(2)O(s) 0^(@)C, 1 bar
Text Solution
|
- For the process H(2)O(l) (1 "bar", 373 K) rarr H(2)O(g) (1"bar", 373 K...
Text Solution
|