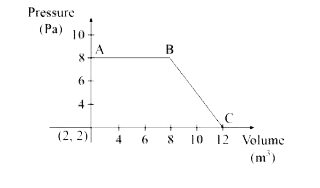
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
VMC MODULES ENGLISH|Exercise JEE ADVANCED (ARCHIVE)|44 VideosTHERMODYNAMICS
VMC MODULES ENGLISH|Exercise LEVEL-2 (NUMERICAL VALUE TYPE)|15 VideosTHERMOCHEMISTRY
VMC MODULES ENGLISH|Exercise JEE ADVANCED (ARCHIVE)|31 VideosTHERMODYNAMICS & THERMOCHEMISTRY
VMC MODULES ENGLISH|Exercise Impeccable|48 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-THERMODYNAMICS-JEE (MAIN ARCHIVE)
- Consider the reversible isothermal expansion of an ideal gas in a clos...
Text Solution
|
- The entropy change associated with the conversion of 1 kg of ice at 27...
Text Solution
|
- An ideal gas undergoes isothermal compression from 5 m^(3) to 1 m^(3) ...
Text Solution
|
- The process with negative entropy change is
Text Solution
|
- Two bolcks to the same metal having same mass and at temperature T1 an...
Text Solution
|
- for a diatomic ideal gas in a closed system , which of the follo...
Text Solution
|
- A process has Delta H= 200 J mol^(-1) and DeltaS=40" JK"^(-1)mol^(-1)....
Text Solution
|
- The standard reaction Gibbs energy for a temperature T is given by ...
Text Solution
|
- The reaction, MgO(s) + C(s) to Mg(s) + CO(g), for which Delta(r)H...
Text Solution
|
- In which case, process will be spontaneous at all temperature?
Text Solution
|
- Among the following, the set of parameters that represents path functi...
Text Solution
|
- Maltose on treatment with dilute HCl gives:
Text Solution
|
- For silver, C(P)(J K^(-1)"mol"^(-1))=23+0.01T. If the temperature (T) ...
Text Solution
|
- Which of the following is not correct for an ideal gas as per first lo...
Text Solution
|
- During compression of a spring the work done is 10 kJ and 2 kJ escaped...
Text Solution
|
- 5 moles of an ideal gas at 100 K are allowed to undergo reversible com...
Text Solution
|
- At constant volume 4 mol of an ideal gas when heated form 300k to 50...
Text Solution
|
- The true statement amongst the following is :
Text Solution
|
- The magnitude of work done by a gas that undergoes a reversible expans...
Text Solution
|
- A ("(l)") to 2 B ( "(g)") Delta U = 2. 1 kcal , Delta...
Text Solution
|