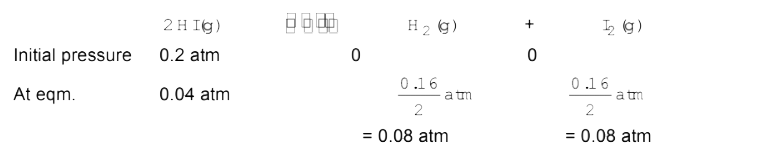Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise Level 0 SA II|6 VideosCHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise Level 0 LA|6 VideosCHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE - G|10 VideosCHEMICAL BONDING-I & II
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|98 VideosCHEMICAL KINETICS
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|52 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-CHEMICAL EQUILIBRIUM-Level 0 SA I
- At a certain temperature and a total pressure of 10^(5) Pa, iodine vap...
Text Solution
|
- Find out the value of K(c ) for each of the following equilibrium from...
Text Solution
|
- Explain why pure liquids and solids can be ignored while writing the e...
Text Solution
|
- A sample of HI(g) is placed in flask at at pressure of 0.2 atm . At eq...
Text Solution
|
- What is the relation between the equilibrium constant of the forward r...
Text Solution
|
- What is the relation between the activity of a gaseous component and i...
Text Solution
|
- In the reaction PCl(5)(g) rarr PCl(3)(g) + Cl(2)(g) PCl(5),PCl(3)"and"...
Text Solution
|