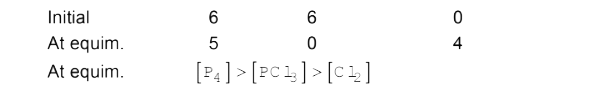A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise Level 2|65 VideosCHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise JEE Main (Archive)|24 VideosCHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise Level 0 LA|6 VideosCHEMICAL BONDING-I & II
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|98 VideosCHEMICAL KINETICS
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|52 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-CHEMICAL EQUILIBRIUM-Level 1
- The compounds A and B are mixed in equimolar proportion to form the pr...
Text Solution
|
- In which of the following reactions, the concentration of product is h...
Text Solution
|
- The equilibrium: P(4)(g)+6Cl(2)(g) hArr 4PCl(3)(g) is attained by ...
Text Solution
|
- Consider the following reaction equilibrium N(2)(g) + 3H(2)(g) hArr...
Text Solution
|
- A reversible reaction is one which :
Text Solution
|
- In a reaction the rate of reaction is proportional to its active mass,...
Text Solution
|
- In the equilibrium,AB(s) rarr A(g) + B(g), if the equilibrium concentr...
Text Solution
|
- According to law of mass action, for the reaction :2A + B rarr Product...
Text Solution
|
- For the system 3A +2B hArr C the expression for equilibrium constant K...
Text Solution
|
- 5 mole of X are mixed with 3 moles of Y. At equilibrium for the reacti...
Text Solution
|
- The equilibrium constant of a reversible reaction at a given temperatu...
Text Solution
|
- For the reaction,Fe(s) + S(s) rarr FeS(s) the expression for equilibr...
Text Solution
|
- For which of the following reaction does the equilibrium constant depe...
Text Solution
|
- On a given condition, the equilibrium concentration of HI , H(2) and ...
Text Solution
|
- The unit of equilibrium constant K(c) for the reaction A+B hArr C woul...
Text Solution
|
- In a reaction A+2B hArr 2C, 2.0 moles of 'A' 3 moles of 'B' and 2.0 m...
Text Solution
|
- In which one of the following gaseous equilibrium, K(p) is less than K...
Text Solution
|
- The equilibrium constant for the reaction N(2)(g)+3H(2)(g)hArrNH(3)(...
Text Solution
|
- For the reaction, 2NO(2)(g) rarr 2NO(g) + O(2)(g), K(c) = 1.8 xx 10^(-...
Text Solution
|
- For the dissociation reaction N(2)O(4) (g)hArr 2NO(2)(g), the degree o...
Text Solution
|