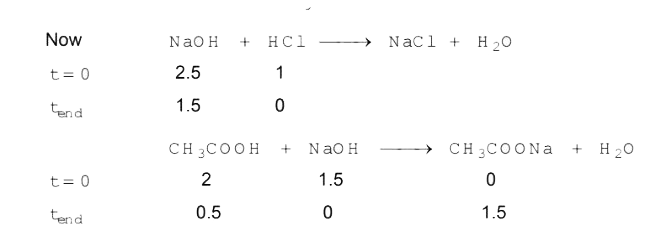
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
IONIC EQUILIBRIUM
VMC MODULES ENGLISH|Exercise JEE ADVANCED( ARCHIVE )|65 VideosIONIC EQUILIBRIUM
VMC MODULES ENGLISH|Exercise Illustration - 1|1 VideosIONIC EQUILIBRIUM
VMC MODULES ENGLISH|Exercise LEVEL 2 NUMERICAL VALUE TYPE|15 VideosINTRODUCTION TO ORGANIC CHEMISTRY
VMC MODULES ENGLISH|Exercise JEE ADVANCED ARCHIVE|81 VideosJEE MAIN - 5
VMC MODULES ENGLISH|Exercise PART II : CHEMISTRY (SECTION - 2)|5 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-IONIC EQUILIBRIUM-JEE MAIN ( ARCHIVE )
- An aqueous solution contains 0.10 M H(2)S and 0.20 M HCl. If the equil...
Text Solution
|
- An aqueous solution contains an unknown concentration of Ba^(2+). When...
Text Solution
|
- Following four solution are prepared by mixing different volumes of Na...
Text Solution
|
- A mixture of 200 mmol of Ca(OH)2 and 3g of sodium sulphate was diss...
Text Solution
|
- 20mL of 0.1M(H(2)SO(4))solution is added to 30mL of 0.2M(NH(4)OH) soul...
Text Solution
|
- If K(sp) of Ag(2)CO(3) is 8 xx 10^(-12), the molar solubility of Ag(2)...
Text Solution
|
- For the equilibrium, 2H(2)O hArr H(3)O^(+) + OH^(-),the value of De...
Text Solution
|
- Consider the following statements A. The chloroplast pigments are fa...
Text Solution
|
- If solubility product of Zr(3)(PO(4))(4) is denotes by K(sp) and its m...
Text Solution
|
- The molar solubility of Al(OH)(3) in 0.2 M NaOH solution is x xx 10^(...
Text Solution
|
- The molar solubility of Cd(OH)(2) is 1.84xx10^(-5)M in water. The exp...
Text Solution
|
- The pH of 0.02MNH(4)Cl solution will be : [Given K(b) (NH(4)OH) = 10^...
Text Solution
|
- In an acid base titration, 0.1 M HCI solution was added to the NaOH so...
Text Solution
|
- 3 g of acetic acid is added to 250 mL of 0.1 M HCl and the solution ma...
Text Solution
|
- Assertion: pH of water increases on increasing temperature. Reason: ...
Text Solution
|
- The solubility product of Cr(OH)(3) at 298 K is 6.0xx10^(-31). The con...
Text Solution
|
- The K(sp) for the following dissociation is 1.6xx10^(-5) PbCl(2) (s) ...
Text Solution
|
- The stoichiometre and solubility product oFIGURE a salt with the solub...
Text Solution
|
- The strength of an aqueous NaOH solution is most accurately determined...
Text Solution
|
- Two solutions, A and B, each of 100 L was made by dissolving 4g of NaO...
Text Solution
|