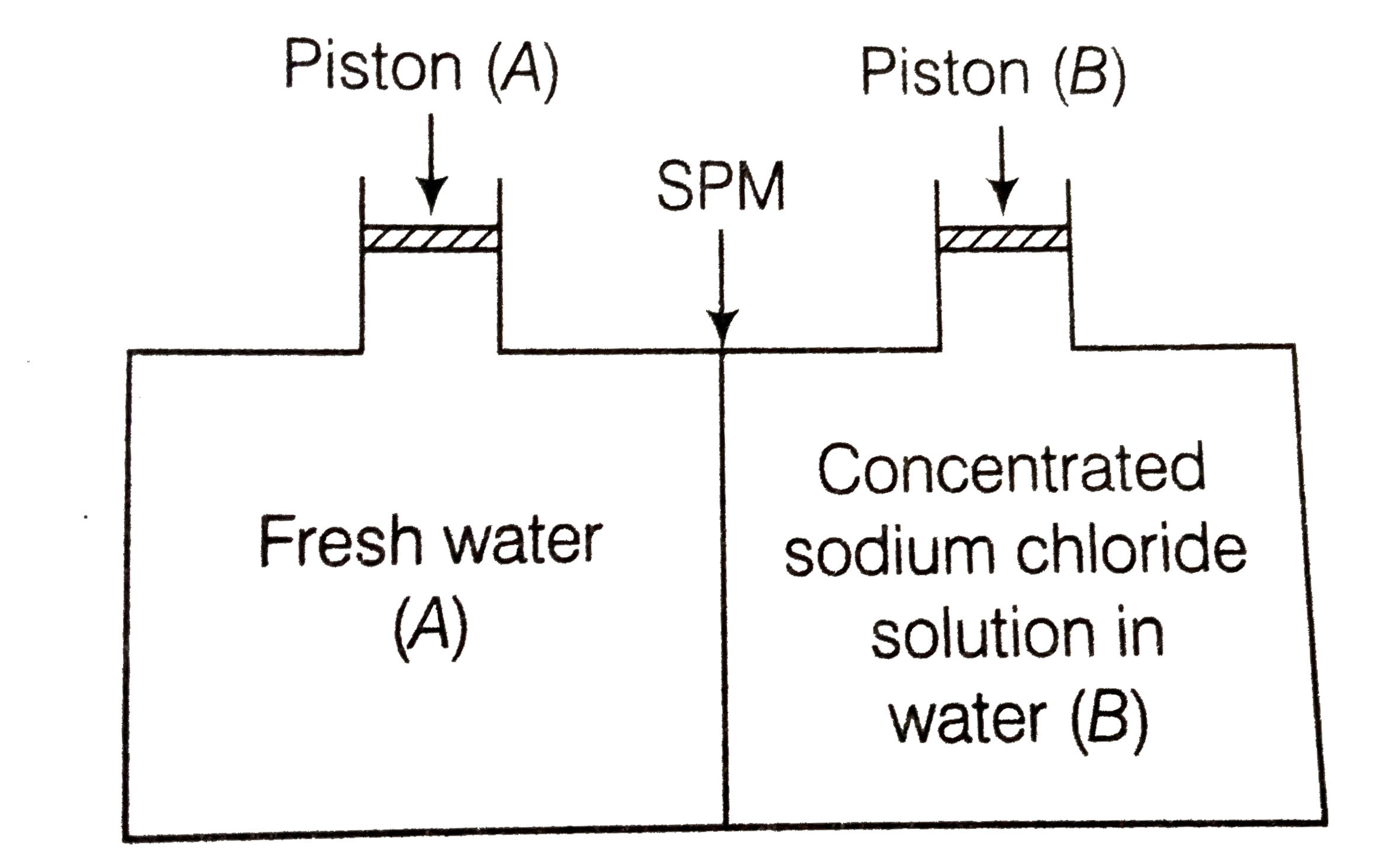
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
THEORY OF SOLUTIONS
VMC MODULES ENGLISH|Exercise JEE Main (Archive)|57 VideosTHEORY OF SOLUTIONS
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|31 VideosTHEORY OF SOLUTIONS
VMC MODULES ENGLISH|Exercise LEVEL 1|75 VideosTHE SOLID STATE
VMC MODULES ENGLISH|Exercise EXERCISE-J|10 VideosTHERMOCHEMISTRY
VMC MODULES ENGLISH|Exercise JEE ADVANCED (ARCHIVE)|31 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-THEORY OF SOLUTIONS-LEVEL 2
- Consider the following vapour pressure-composition graph, SP is equal ...
Text Solution
|
- For a non-volatile solute
Text Solution
|
- Consider the figure and mark the correct option.
Text Solution
|
- At 40^(@)C, the vapour pressures in torr, of methyl alcohol and ethyl ...
Text Solution
|
- What mass of urea be dissolved in 171 g of water so as to decrease the...
Text Solution
|
- Increasing amount of solid Hgl(2) is added to 1 L of an aqueous soluti...
Text Solution
|
- Equimolal solutions KCl and compound X in water show depression in fre...
Text Solution
|
- CNS^(ө) ions give red colour with Fe^(3+) ions in aqueous solution as:...
Text Solution
|
- Consider the two solutions: I: 0.5 M NaCl aqueous solution at 25^(@)...
Text Solution
|
- Which pair(s) of liquids on mixing is/are expected to show no net volu...
Text Solution
|
- Assertion (A): The molecular mass of polymers cannot be calculated usi...
Text Solution
|
- Assertion (A): Camphor is used as a solvent in the determination of th...
Text Solution
|
- The relative decrease in VP of an aqueous glucose dilute solution is f...
Text Solution
|
- Which has maximum osmotic pressure at temperature T? a. 100 mL of 1...
Text Solution
|
- A mixture of volatile component A and B has total vapour pressure (in ...
Text Solution
|
- 1 mol benzene (P^(@)("benzene")=42 mm) and 2 mol toluence (P^(@)("to...
Text Solution
|
- When non-volatile solute is added to a pure solvent, the:
Text Solution
|
- An aqueous solution has freezing point at -2.55^(@)C. What is its boi...
Text Solution
|
- Among the following, the solution which shows the lowest osmotic press...
Text Solution
|
- A 0.1 M solution of glucose (molecular weight 180 g "mol"^(-1)) and a...
Text Solution
|