A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
THEORY OF SOLUTIONS
VMC MODULES ENGLISH|Exercise JEE Main (Archive)|57 VideosTHEORY OF SOLUTIONS
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|31 VideosTHEORY OF SOLUTIONS
VMC MODULES ENGLISH|Exercise LEVEL 1|75 VideosTHE SOLID STATE
VMC MODULES ENGLISH|Exercise EXERCISE-J|10 VideosTHERMOCHEMISTRY
VMC MODULES ENGLISH|Exercise JEE ADVANCED (ARCHIVE)|31 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-THEORY OF SOLUTIONS-LEVEL 2
- Two equimolar solutions have osmotic pressures in the ratio of 2 : 1 a...
Text Solution
|
- Human blood gives rise to an osmotic pressure of approximately 7.65 at...
Text Solution
|
- Osmotic pressure of insulin solution at 298 K is found to be 0.0072atm...
Text Solution
|
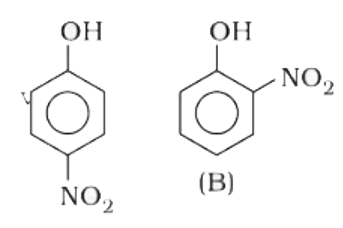
- Out of the compounds below, the vapour pressure of (B) at a particular...
Text Solution
|
- How much urea must be dissolved in 10^(-2)m^(3) water to yield a press...
Text Solution
|
- Acetic acid exists in benzene solution in the dimeric form. In an actu...
Text Solution
|
- Calculate the mass of a non-volatile solute (molar mass 40 g mol^(-1))...
Text Solution
|
- 1000gm of sucrose solution in water is cooled to -0.5^(@)C. How much ...
Text Solution
|
- The depression in freezing point of 0.01 m aqueous CH(3)CooH solution ...
Text Solution
|
- When 20 g of naphthoic acid (C(11)H(8)O(2)) is dissolved in 50 g of be...
Text Solution
|
- 75.2 g of phenol is dissolved in a solvent of Kf=14. If the depression...
Text Solution
|
- A storage battery contains a solution of H(2)SO(4) 38% by weight. At t...
Text Solution
|
- Calculate the apparent degree of ionisation of an electrolyte MX(2) i...
Text Solution
|
- The vapour pressure of a solution of a non-volatile electrolyte B in a...
Text Solution
|
- The relative lowering of the vapour pressure of an aqueous solution co...
Text Solution
|
- A 5 per cent aqueous solution by mass of a non-volatile solute boils a...
Text Solution
|
- One gram of serum albumin dissolved in 1000cm^(3) of water gives a sol...
Text Solution
|
- Density of a 2.05 M solution of acetic acid in water is 1.02 g/mL. The...
Text Solution
|
- The freezing point of 0.08 molal NaHSO(4) is -0.345^(@)C. Calculate th...
Text Solution
|
- Freezing point of 0.2 M KCN solution is -0.7^(@)C. On adding 0.1 mole ...
Text Solution
|