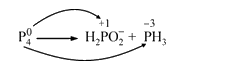Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STOICHIOMETRY-II
VMC MODULES ENGLISH|Exercise LEVEL (2)|65 VideosSTOICHIOMETRY-II
VMC MODULES ENGLISH|Exercise JEE Main (Archive)|21 VideosSTOICHIOMETRY-II
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|43 VideosSTOICHIOMETRY - I
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|31 VideosSTRUCTURE OF ATOM
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE-F|7 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-STOICHIOMETRY-II-LEVEL (1)
- When H2S reacts with halogens, halogens are
Text Solution
|
- Among NH(3), HNO(3), N(2) and Mg(3)N(2) , the number of molecules havi...
Text Solution
|
- The reaction , P(4) + 3NaOH + 3H(2)O to 3NaH(2)PO(2) + PH(3) is an...
Text Solution
|
- The oxidation number of oxygen in KO(3),Na(2)O(2 respectively are:
Text Solution
|
- The oxidation state of sulphur in sodium tetrathionate (Na(2)S(4)O(6))...
Text Solution
|
- The oxidation state of A, B, and C in a compound are +2, +5, and -2, r...
Text Solution
|
- Which of the following chemical reactions depicts the oxidizing behavi...
Text Solution
|
- The oxidation sates of iodine in HIO(4), H(3)IO(5) and H(5)IO(6) are r...
Text Solution
|
- In which of the following compounds, the oxidation number of iodine is...
Text Solution
|
- Oxidation number of 'N' in N(3)H (hydrazoic acid) is :-
Text Solution
|
- MnO(4)^(-) is a good oxidising agent in different mediums changing to ...
Text Solution
|
- Which of the following acts as an oxidising as well as reducing agent ...
Text Solution
|
- What is the oxidation state of P in Ba (H(2)PO(2))(2) ?
Text Solution
|
- Arrange the following in the increasing order of oxidation state of Mn...
Text Solution
|
- In which of the following reactions hydrogen is acting as an oxidising...
Text Solution
|
- In that compounds KMnO(4) and K(2)Cr(2)O(7) the highest oxidation stat...
Text Solution
|
- Which conversion is an oxidation?
Text Solution
|
- The oxidation state of nitrogen is highest in
Text Solution
|
- In a reaction KCl is converted into KClO4. Change in oxidation number ...
Text Solution
|
- In which of the following reaction, oxidation number of Cr has been af...
Text Solution
|