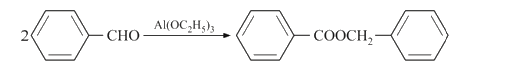
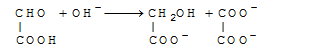A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STOICHIOMETRY-II
VMC MODULES ENGLISH|Exercise JEE Main (Archive)|21 VideosSTOICHIOMETRY-II
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|43 VideosSTOICHIOMETRY-II
VMC MODULES ENGLISH|Exercise LEVEL (1)|75 VideosSTOICHIOMETRY - I
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|31 VideosSTRUCTURE OF ATOM
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE-F|7 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-STOICHIOMETRY-II-LEVEL (2)
- The reaction, 3Br(2)+6CO(3)^(2-)+3H(2)Oto5Br^(-)+BrO(3)^(-)+6HCO(3)^(-...
Text Solution
|
- Which of the following statements is/are true if 1 mol of H(3)PO(x) is...
Text Solution
|
- Which is not a disproportionation reaction :
Text Solution
|
- Assertion : The reaction of ammonia solution with calomel is a disprop...
Text Solution
|
- Assertion : When SnCl(2) solution is added to HgCl(2) solution, a milk...
Text Solution
|
- In a balanced equation H(2)SO(4)toxHItoH(2)S+yI(2)+zH(2)O The valu...
Text Solution
|
- Pyrolusite is the main ore of manganese in which it is present as It...
Text Solution
|
- Pyrolusite is the main ore of manganese in which it is present as It...
Text Solution
|
- Pyrolusite is the main ore of manganese in which it is present as It...
Text Solution
|
- Pyrolusite is the main ore of manganese in which it is present as It...
Text Solution
|
- Pyrolusite is the main ore of manganese in which it is present as It...
Text Solution
|
- In the permanganate titration, the solution of reductant is always mad...
Text Solution
|
- If 1.34g Na(2)C(2)O(4)is dissolved in 500 mL of water and this solut...
Text Solution
|
- In the titration of NaOH and HCl, which of the following indicator wil...
Text Solution
|
- 10 g of oxalate was dissolved in 300 mL of solution. This solution req...
Text Solution
|
- 80 mL of M/24 K(2)Cr(2)O(7) oxidises 22.4 mL H(2)O(2) solution. Find v...
Text Solution
|
- Five moles of ferric oxalate are oxidise by how much mole of KMnO(4) i...
Text Solution
|
- Intramolecular redox (NH(4))(2)Cr(2)O(7) rarr N(2) + Cr(2)O(3) + 4H(2)...
Text Solution
|
- How many gram of I(2) are present in a solution which requires 40 mL o...
Text Solution
|
- What volume of 2 N K(2)Cr(2)O(7) solution is required to oxidise 0.81...
Text Solution
|