A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
VMC MODULES ENGLISH|Exercise Level-2 ( Numerical Value Type for JEE Main )|15 VideosCHEMICAL KINETICS
VMC MODULES ENGLISH|Exercise JEE Main (Archive)|56 VideosCHEMICAL KINETICS
VMC MODULES ENGLISH|Exercise Level-1|75 VideosCHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE - G|10 VideosCHEMICAL THERMODYNAMICS
VMC MODULES ENGLISH|Exercise IN - CHAPTER EXERCISE - L|10 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-CHEMICAL KINETICS -Level-2
- The activation energy of exothermic reaction ArarrB is 80 kJ "mol"^(-1...
Text Solution
|
- For a gaseous reaction, following data is given: ArarrB, k(1)= 10^(1...
Text Solution
|
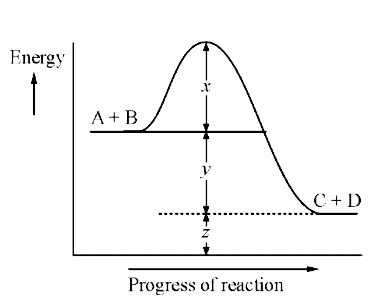
- Given the following diagram for the reaction A+B rarr C+D The enthalpy...
Text Solution
|
- The rate constant of a first order reaction at 27^@C is 10–3 min–1. Th...
Text Solution
|
- For the reaction 2NH(3) to N(2) + 3H(2), If - (d[NH(3)])/(dt) = k(!)...
Text Solution
|
- A catalyst lowers the enrgy of activation by 25%, temperature at which...
Text Solution
|
- In the following graphical representation for the reaction A rarr B, t...
Text Solution
|
- For a reaction of reversible nature, net rate is ((dx)/(dt))=k(1)[A][B...
Text Solution
|
- Rate constant k varies with temperature by equation, log k (min+^(-1))...
Text Solution
|
- If a homogeneous catalytic reaction follows three alternative paths A,...
Text Solution
|
- Two different first order reactions have rate consants k(1) and k(2) "...
Text Solution
|
- Consider the following statement : 1 The rate of reaction is always ...
Text Solution
|
- In the reaction A+BrarrC+D, the rate ((dx)/(dt)) when plotted against ...
Text Solution
|
- Initial concentration of reactant for nth order reaction is 'a'. Which...
Text Solution
|
- According to collision theory, which of the following is the criteria ...
Text Solution
|
- For the first-order reaction (C=C(0)e^(-k(1)^(t))) and T(av)=k(1)^(-1)...
Text Solution
|
- A reactant (A) forms two products A overset (k(1))rarr B, Activation...
Text Solution
|
- Consider the reaction : 2H(2)(g)+2NO(g)rarrN(2)(g)+2H(2)(g) The r...
Text Solution
|
- Which of the following is correct?
Text Solution
|
- Consider the following reaction. The reaction is first order in each ...
Text Solution
|