Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-CHEMICAL KINETICS -JEE Advanced (Archive)
- The rate constant of a reaction depends upon
Text Solution
|
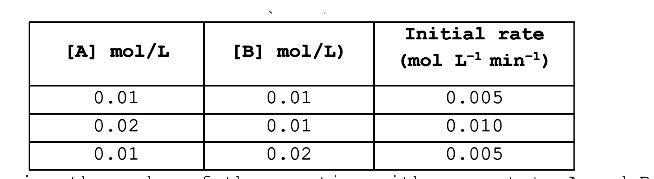
- Rate of reaction, A+B rarr products is given below as a function of di...
Text Solution
|
- A first order reaction is 20% complete in 10 min. Calculate (a) the sp...
Text Solution
|
- The specific rate constant of a first order reaction depends on the
Text Solution
|
- A catalyst is a substance which :
Text Solution
|
- A catalyst :
Text Solution
|
- While studying the decompoistion of gaseous N(2)O(5), it is observed t...
Text Solution
|
- The rate of chemical change is directly proportional to …………… .
Text Solution
|
- For a first order reaction, the rate of the reaction doubled as the co...
Text Solution
|
- The hydroliss of ethyl acetate in………… medium is a…………..order reaction.
Text Solution
|
- A first order gas reaction has k = 1.5 xx 10^(-6) s^(-1) at 200^(@)C. ...
Text Solution
|
- A first order reaction is 50% complete in 30 minutes at 27^(@)C and in...
Text Solution
|
- In a Arrhenius equation for a certain reaction, the values of A and E(...
Text Solution
|
- The decompoistion of N(2)O(5) according to the equation 2N(2)O(5)(g) r...
Text Solution
|
- Two reaction, (I)A rarr Products and (II) B rarr Products, follow firs...
Text Solution
|
- A first order reaction A rarr B requires activation energy of 70 kJ mo...
Text Solution
|
- The gas phase decomposition of dimethyl ether follows first order kine...
Text Solution
|
- The progress of the reaction, A ⇌ nB with time, is presented in figure...
Text Solution
|
- For the reaction N(2)(g)+3H(2)(g) rarr 2NH(3)(g), under certain condit...
Text Solution
|
- From the following data for the reaction between A and B Calculat...
Text Solution
|