A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise ENABLE|49 VideosCHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise EFFICIENT|50 VideosCHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|28 VideosCHEMICAL BONDING-I & II
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|98 VideosCHEMICAL KINETICS
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|52 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-CHEMICAL EQUILIBRIUM-FUNDAMENTAL
- In a reaction A+BhArrC+D the rate constant of forward reaction & backw...
Text Solution
|
- K1and K2 are equilibrium constant for reactions (i) and (ii) N2(g) +...
Text Solution
|
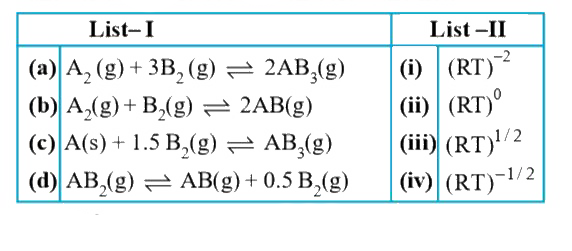
- Match List - I (hypothetical reactions) with List - II (ratio (K(p))/(...
Text Solution
|
- log.(K(P))/(K(C))+logRT=0. For which of the following reaction is this...
Text Solution
|
- For reaction 2NO(g)hArr N(2)(g)+O(2)(g) degree of dissociation of N(2)...
Text Solution
|
- If the equilibrium constant of the reaction 2HIhArr H(2)+I(2) is 0.25,...
Text Solution
|
- In the 500 ml flask following reaction takes place H(2)(g)+I(2)(g)hArr...
Text Solution
|
- At equilibrium degree of dissociation of SO(3) is 50% for the reaction...
Text Solution
|
- At 250^(@)C and one atmosphere PCl(5) is 40% dissociated The equilibri...
Text Solution
|
- For the reaction I(2)(g) hArr 2I(g), K(c) = 37.6 xx 10^(-6) at 1000K ....
Text Solution
|
- Consider the reaction A(g)+B(g) hArr C(g)+D(g) Which occurs in one...
Text Solution
|
- For reaction, PCl(3)(g)+Cl(2)(g) hArr PCl(5)(g) the value of K(c) ...
Text Solution
|
- For the system A(g)+2B(g) hArr C(g) the equilibrium concentration is ...
Text Solution
|
- For a very small extent of dissociation of PCl(5) into PCl(3) and Cl(2...
Text Solution
|
- 1 mol of N(2) is mixed with 3 mol of H(2) in a litre container. If 50%...
Text Solution
|
- {:("For the reaction",PCl(5)hArrPCl(3)+Cl(2)),("Initial moles are"," a...
Text Solution
|
- For reaction : H(2)(g)+I(2)(g) hArr 2HI(g) at certain temperature, the...
Text Solution
|
- At temperature T, a compound AB2(g) dissociates according to the react...
Text Solution
|
- 2 is the equilibrium constant for the reaction A(2)+B(2)hArr 2AB at a ...
Text Solution
|
- For the reaction N(2)+O(2)hArr 2NO at equilibrium number of moles of N...
Text Solution
|