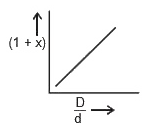
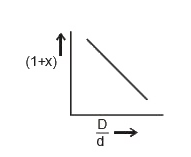A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise ENABLE|49 VideosCHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise EFFICIENT|50 VideosCHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|28 VideosCHEMICAL BONDING-I & II
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|98 VideosCHEMICAL KINETICS
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|52 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-CHEMICAL EQUILIBRIUM-FUNDAMENTAL
- The value of K for H(2)(g)+CO(2)(g)hArr H(2)O(g)+CO(g) is 1.80 at 1000...
Text Solution
|
- In a 20 litre vessel at 300 K initially, 1 mole of CO, H(2)O, CO(2) ar...
Text Solution
|
- A(3)(g)hArr 3A(g), In the above reaction, the initial conc. Of A(3) is...
Text Solution
|
- PCl(5)hArrPCl(3)+Cl(2) in the above reaction the partial pressure of P...
Text Solution
|
- PCl(5)(g)hArr PCl(3)(g)+Cl(2)(g), In above reaction, at equilibrium co...
Text Solution
|
- In 5 litre container 1 mole of H(2) and 1 mole of I(2) are taken initi...
Text Solution
|
- SO(2)+(1)/(2)O(2)hArrSO(3), for the above reaction, if 'a' and 'b' mol...
Text Solution
|
- In an experiment the equilibrium constant for the reaction A+BhArr C+D...
Text Solution
|
- In which of the following reaction, the yield of the products does not...
Text Solution
|
- In the formation of nitric acid, N(2) and O(2) are made to combine. Th...
Text Solution
|
- For the the manufacture of ammonia by the reaction N(2)+3H(2)hArr 2NH(...
Text Solution
|
- Which of the following equilibria will proceed to farthest completion?
Text Solution
|
- The concentration of a pure solid or liquid phase is not include in th...
Text Solution
|
- In the system A((s))hArr2B((g))+3C((g)), if the concentration of C at ...
Text Solution
|
- In the equilibrium,AB(s) rarr A(g) + B(g), if the equilibrium concentr...
Text Solution
|
- The vapour density of N(2)O(4) at a certain temperature is 30. Calcula...
Text Solution
|
- In an equilibrium reaction for which DeltaG^(ɵ)=0, the equilibrium con...
Text Solution
|
- The vapour density of Pcl(5) is 104.16 but when heated to 230^(@)C, it...
Text Solution
|
- For the reaction : AhArr nB, if 'a' mole of A is taken initially and '...
Text Solution
|
- In the dissociation of N(2)O(4) " into "NO(2), (1+alpha) values with t...
Text Solution
|