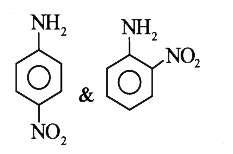
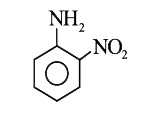
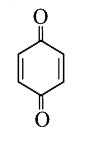
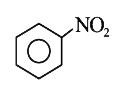
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
AMINES
VMC MODULES ENGLISH|Exercise EFFICIENT|49 VideosAMINES
VMC MODULES ENGLISH|Exercise IMPECCABLE|48 VideosAMINES
VMC MODULES ENGLISH|Exercise IN CHAPTER EXERCISE H|10 VideosALDEHYDES,KETONES & CARBOXYLIC ACIDS
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE-M|10 VideosATOMIC STRUCTURE
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive )|67 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-AMINES-ENABLE
- Ethyl isocyanate on hydrolysis with water partially gives
Text Solution
|
- Reaction of aniline with benzaldehyde is
Text Solution
|
- Aniline when treated with K2 Cr2 O7 in conc H2 SO4 gives :
Text Solution
|
- Which of the following is the least basic?
Text Solution
|
- the reduction on benzene diazonium chloride with SnCl2 and HCI a...
Text Solution
|
- most basic out of these is :
Text Solution
|
- Following reaction gives two products . Write the structures of the pr...
Text Solution
|
- The bromination of aniline in presence of water produces :
Text Solution
|
- The order of basicity of amines in gaseous state is-
Text Solution
|
- Examine the following two structures for the anilinium ion and choose...
Text Solution
|
- The reduction of a nitrile by LiAlH( 4 ) produes :
Text Solution
|
- Which of the following compounds will dissolve in an alkali solution a...
Text Solution
|
- In the series of reactions CH3 COOH underset(P2O5)overset(NH3)to A A...
Text Solution
|
- Ethyl amine reacts with nitrosyl chloride to form
Text Solution
|
- Treatment of ammonia with excess ethyl chloride will yield
Text Solution
|
- A colourless , odourless and non - combustible gas is liberated...
Text Solution
|
- Heating of [CH3 CH2 CH2CH(CH3 )N^(+)(CH3)3 OH ^- gives the major...
Text Solution
|
- Secondary amine forms yellow oily liquid nitrons acid , which ...
Text Solution
|
- Consider the following reaction , C(6)H(5)NH(2)+CHCl(3)+KOHoverset(D...
Text Solution
|
- When aniline is treated with benzene diazonium chloride at low ...
Text Solution
|