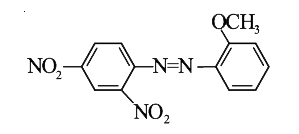
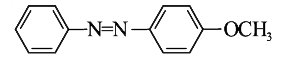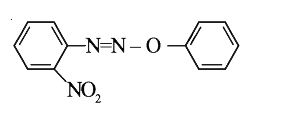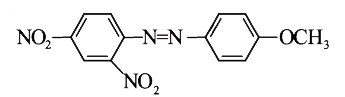A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
AMINES
VMC MODULES ENGLISH|Exercise IMPECCABLE|48 VideosAMINES
VMC MODULES ENGLISH|Exercise ILLUSTRATION|25 VideosAMINES
VMC MODULES ENGLISH|Exercise ENABLE|45 VideosALDEHYDES,KETONES & CARBOXYLIC ACIDS
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE-M|10 VideosATOMIC STRUCTURE
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive )|67 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-AMINES-EFFICIENT
- Allyl isocyanide contains sigma and pi bonds are-
Text Solution
|
- Nitrobenzenen can be prepared from benzene by using a mixture of conc ...
Text Solution
|
- Which one of the following statements on the nitration of aromatic com...
Text Solution
|
- Benzene diazonium chloride on reaction with phenol in weakly basic med...
Text Solution
|
- The product not obtained in the following reaction , CH (3) - NO2...
Text Solution
|
- A positive carbylamine test is given by:
Text Solution
|
- Among the following the strongest base is
Text Solution
|
- Select the most basic compound.
Text Solution
|
- the correct order of basicities of the following compounds is
Text Solution
|
- Which of the following represent the given mode of hybridization sp^(2...
Text Solution
|
- In the compound given below the correct order of actidity...
Text Solution
|
- Which of the following species contains 5 H atoms , sp ^2 hybr...
Text Solution
|
- Which of the following does not produce salt with hydrochloric ...
Text Solution
|
- Which is true about aniline ?
Text Solution
|
- Which of the following compound will not show tautomerism ?
Text Solution
|
- CH(3)CH(2)NH(2) overset((CH(3)CO)(2))underset(Delta) to A+B, (A) and (...
Text Solution
|
- 2-4 dinitroaniline is treated with NaNO(2) and dilute HCI at 280...
Text Solution
|
- Which of the following compound exists as a dipolar ion ?
Text Solution
|
- Predict the final product (C ) in the following sequence of re...
Text Solution
|
- CH(3) CH (CH(3))CH(2)NH(2) overset( NaNO(2)//HCI ) to (A) overset( a...
Text Solution
|